To get to the bottom of what happened nine years ago this week, when the Deepwater Horizon (DWH) oil rig exploded, killing 11 people and triggering the worst environmental disaster in U.S. history, you need to go deep.
Real deep.
You need to go, in fact, thousands of meters below the surface of the ocean, where pressures are more than 2,000 pounds per square inch, temperatures are near freezing and darkness reigns around the clock.
That is the depth to which organic geochemist Isabel Romero and her colleagues have plunged in their search for tens of millions of gallons of oil that gushed from the DWH rig 42 miles off the Louisiana coast and was never recovered.
That sunny, spring day marked the beginning of an 87–day hemorrhage that eventually released as much as 200 million gallons of crude oil into the Gulf of Mexico. About a fifth was recovered from the wellhead. Of the rest, some was burned, some evaporated, some was skimmed from the surface. Some oil formed a slick the size of Missouri; some floated under the surface in a “plume” covering an area larger than Ireland.
A substantial amount went missing in action.
Exactly where did all that oil go, and what became of it? That’s what Romero came to the National MagLab this month to better understand.
“The concept of things disappearing is not quite right,” said Romero, a research associate at the University of South Florida’s College of Marine Science. “Things don’t disappear. They transform into something else — or go someplace else.”
And when they transform, Romero knew, they often become more dangerous to marine life, water quality and the communities living nearby.
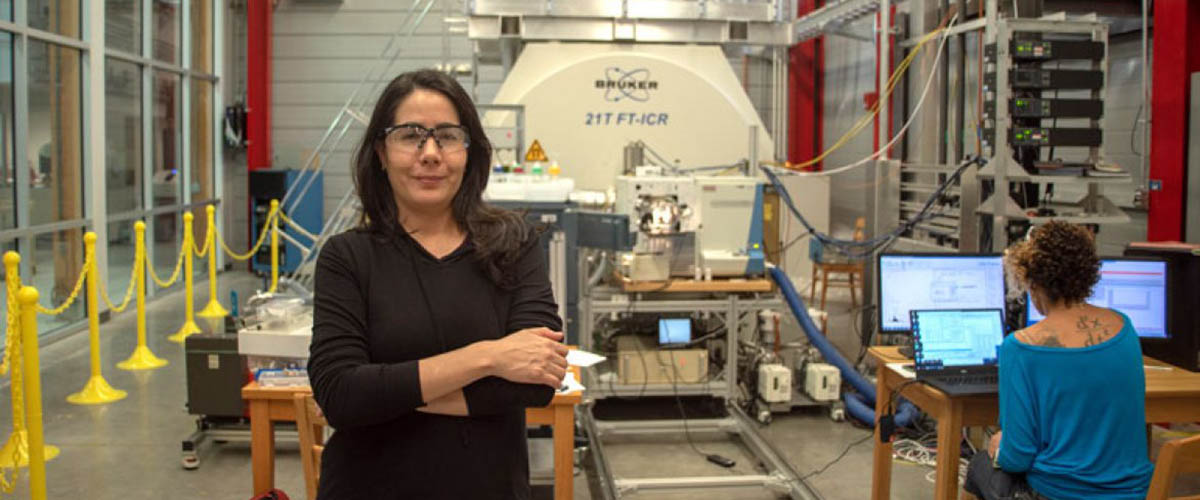
Seeking details on those transformations, Romero recently drove up to the MagLab’s Tallahassee-based Ion Cyclotron Resonance (ICR) Facility from St. Petersburg, Fla., hauling a cargo of 50 samples of highly complex molecules. At the lab, she knew she would find instrumentation and scientists with world-renowned expertise in studying oil to help with this task.
Her study is funded by the Gulf of Mexico Research Initiative (GoMRI), a decade-long, $500-million effort now entering its final year and bankrolled by BP, the oil company that operated the DWH rig. While most DWH-related research has focused on the disaster’s impact along more than 1,300 miles of coastline, Romero and her collaborators from The University of Southern Mississippi, Florida State University and Eckerd College have looked much farther afield — or rather, farther a-sea. Most of the samples Romero brought with her were collected from the northern Gulf’s sea floor. But she also brought samples from a much older Gulf spill, hoping that an earlier disaster would shed light on the more recent spill’s long-term impact.
That sinking feeling

Romero sorts through sediment samples her team collected from the Gulf of Mexico.
Image credit: Stephen Bilenky
The DWH spill was a made-for-TV disaster (and in fact inspired a 2016 movie starring Mark Wahlberg). As oil gushed into the Gulf from one day, one week, one month to the next, video of oil-drenched pelicans, burning oil slicks and tarball-strewn beaches played on screens around the world.
But a disaster was also taking place out of sight, well below the shadow of the oil slick. Although oil is less dense than water, it doesn’t float all the time.
“Oil in the ocean can sink,” Romero said. “But it has to be weathered and have particles attached to make it heavy and sink.”
Thanks in large part to the mighty Mississippi, there is no shortage of such particles in the Gulf, both biological and mineral, onto which oil residues could hitch a ride to the sea floor. A lot — as much as 47 percent of the unrecovered oil, Romero has estimated — drifted all the way to the bottom.
The National Oceanic and Atmospheric Administration had collected sea floor sediment samples in the area below the DWH slick. But Romero and her colleagues believed the oil had spread well beyond. After all, the sea floor has canyons, watersheds and other topographic features like those found on land, which can affect circulation and transport of sediment.
As part of their GoMRI study, Romero and her colleagues boarded a research vessel in 2018 to collect samples from the ocean floor in a watershed southeast of the slick. They worked around the clock and were interrupted by a rough storm, but eventually made it home a week later with sediment samples from 30 sites.
Retrieving the samples was just the beginning. The team then had to figure out what was in them. Were there traces of oil? If so, where did that oil originate? How did its chemistry vary based on the specific depth at which it was found? Had DWH oil, in fact, traveled so far from the wellhead? How had it changed — and what did those molecular-level changes mean for the environment?
Morphing molecules

Romero fine-tunes the instrumentation for her experiment on the world-record 21-tesla FT-ICR mass spectrometer.
Image credit: Stephen Bilenky
Crude oil is already the most chemically complex substance on Earth. A drop of it can contain hundreds of thousands of different kinds of carbon-packed molecules, or hydrocarbons, making it a challenge to analyze.
It gets even trickier when hydrocarbons change due to exposure to the sun, hungry microbes and other variables, each leaving its molecular mark.
Those marks aren’t easy to see. Like a camouflaged octopus, oil in the environment can hide in plain sight. Romero picked up her clear bottle of drinking water and shook it for effect.
“These sediment samples can have oil and they don’t smell,” she said. “Oil residues at depth don’t have any color, they are transformed.”
The “transformation products,” as they are called, can have different chemical properties than the original compounds, and even higher toxicity. Under the right conditions, a harmless compound can shape-shift into a dangerously different form, like an evil Marvel Skrull.
“Some of the compounds become very toxic,” said Romero. “And we’re targeting those as well.”
Romero analyzed her sediment samples at USF. In previous research, she had already identified specific toxic compounds that occur in DWH oil after it degrades in the environment. She looked for those compounds in her samples using a gas chromatographer-mass spectrometer. Mass spectrometers are instruments that decode the chemical composition of substances by, essentially, weighing the molecules, each of which has a unique molecular mass. As she had predicted, she found the toxic compounds in the new samples — evidence that DWH oil had travelled further than previous studies had shown.
But Romero knew she was seeing just part of the problem: After all, she was searching only for specific compounds. Also, her mass spectrometer couldn’t detect most of the thousands of other compounds in the sediment, many of which are transformation products of oil. She was looking through a peephole, but wanted to fling the door open to see the whole picture.
So she loaded up a rented Nissan with her samples and headed to the MagLab.
Door wide open

Romero reviews the data, which included a far wider range of compounds than she had been able to identify on other instruments.
Image credit: Stephen Bilenky
MagLab chemist Amy McKenna was waiting.
As world leaders in the study of petroluem, McKenna and her colleagues at the MagLab’s ICR Facility have processed hundreds of oil samples over the years, including from some of the worst environmental disasters in the U.S.: Cosco Busan off the San Francisco coast (2007); Kalamazoo River in Michigan (2010); Texas City, Tex. (2014); and the Taylor spill in the Gulf of Mexico, ongoing since 2004.
And, of course, DWH samples.
“We have hundreds of samples that have been collected on beaches, on sand patties, on floating pieces of Styrofoam, in shrimp cakes,” McKenna said. “We have a lot of that stuff.”
But they had never seen sediment from the bottom of the ocean with DWH oil, and McKenna encouraged Romero to bring her samples to the MagLab. Romero had sliced them into 2-mm-thick layers, each containing a trove of data on how the oil residues had transformed at each particular depth.

MagLab chemist Amy McKenna.
Image credit: Dave Barfield
“Some oil has gone up to the surface and gone back down and settled on the bottom of the ocean," McKenna explained. "Some was entrained in the water column and never made it fully to the surface, then settled back down. Some made it all the way to the surface, floated on the ocean, and was deposited on the beaches.”
Every scenario results in a very different complex mixture of compounds. But like a pirate’s longlost treasure chest, the booty was only valuable if you could get to it.
McKenna felt sure, given the MagLab’s expertise in petroleum analysis and its world-record 21-tesla (21T) Fourier transform-ICR (FT-ICR) mass spectrometer, that she could help Romero find the treasure.
The instruments Romero had previously used could detect molecules with an atomic mass of up to 400 daltons (or Da, the unit for atomic mass). Anything heavier was undetectable. But when Romero used the 21T, that window was flung open, revealing molecules with molecular masses upwards of 2,000 Da.
“With the FT-ICR method, you look at the very high molecular masses,” Romero said. “You look at what has been transformed, the complex mixture that reached the bottom of the ocean, the chemical signature of the organic matter after multiple chemical and biological transformation processes.”
After a week at the lab, Romero returned home with lots of new data that will take time to process, her eyes opened to new research possibilities.
“No one has done this work in the deep sea the way that we are targeting now with FT-ICR,” said Romero, who hopes the data will help pioneer new lines of scientific research with McKenna at the MagLab.
Unfortunately, the picture isn’t pretty. In other ongoing research, Romero identified transformation products from weathered oil that were 100 times more toxic than before they had changed.
That makes it really important to find solutions, leading Romero to some forensic chemistry. In addition to her DWH samples, she also brought samples from the southern Gulf to the MagLab. That’s where, 40 years ago this June, the Ixtoc-1 spill occurred, dumping tens of millions of gallons of oil off the Mexican coast.
“We’re taking a new look at old environmental disasters and old oil spills to see if we can actually understand what happened long-term at the molecular level,” said McKenna. “That’s going to help us provide information to the communities that are making decisions whenever a spill happens. Because there’s going to be another one. It’s not a matter of if, but when.”
By Kristen Coyne