As she relaxed at home one Saturday in late 2016, Nasim Pica didn’t expect the National Geographic documentary she decided to watch to end up redirecting her research. "I was just browsing," she recalls, taking a break from finishing her Ph.D. in environmental engineering at Colorado State University (CSU). When the documentary turned its focus to polar bears, however, Pica, who studies water contamination, learned something alarmingly relevant to her work: Scientists had found long-lasting, toxic chemicals in the blood of the largest bears on Earth, despite the fact that they lived so far from where those chemicals are made or used.
Following the trail of PFAS (per- and polyfluoroalkyl substances) would lead Pica to the National High Magnetic Field Laboratory (National MagLab), whose powerful instruments help scientists like her understand the environmental threat PFAS pose.
"I started looking at the [scientific] literature," on PFAS, Pica recalls, "and I learned nearly all humans have them in their blood plasma too." The chemicals are attracting increased attention as more scientists find them in drinking water and cow's milk and as government officials struggle to understand and address the compounds. "Dark Waters," a new film about the chemicals starring Mark Ruffalo, is drawing widespread scrutiny to the issue.

Released in November 2019, the movie "Dark Waters," based on a true story, stars Mark Ruffalo as an attorney who fights chemical companies' use of "forever chemicals."
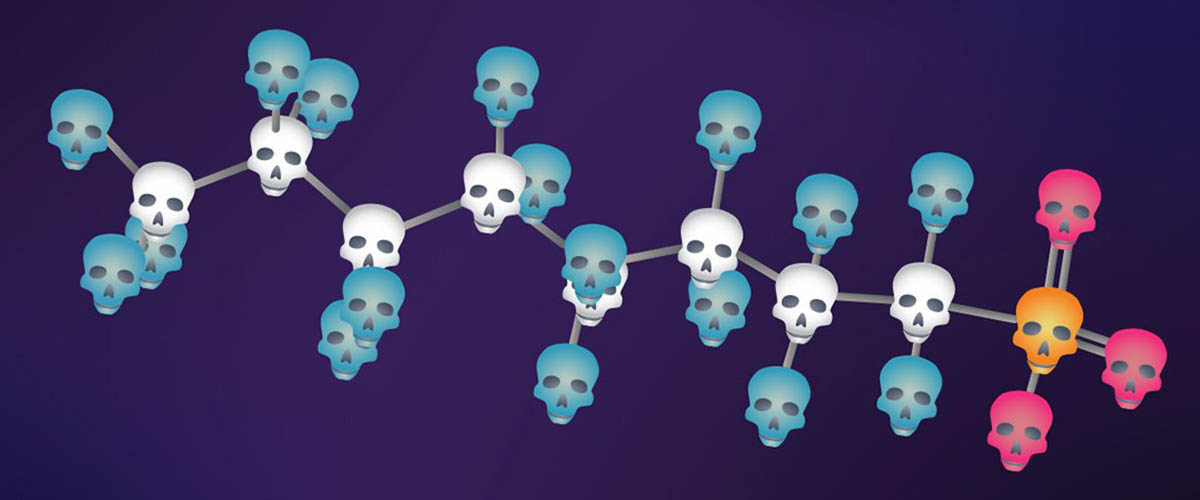
PFAS are a diverse family of molecules, all of which have many fluorine atoms (thus "Perfluoridated" or "Polyfluoridated," accounting for the "PF" in the acronym) attached to a string of carbon atoms called an alkyl chain (thus "Alkylated Substances," the "AS"). The fluorine-carbon (FC) bond is exceptionally strong, making these molecules useful for engineering slick surfaces, from nonstick pans to pizza boxes and compostable food containers. And since the molecules disrupt water's surface tension, helping it foam up, they're a common component of firefighting foams, which smother burning surfaces more effectively than water alone. But the strength of the FC bond means very little can break PFAS down, earning them the foreboding moniker, "forever chemicals."
PFAS enter groundwater or the ocean from many sources, including chemical plants, firefighting training on military bases and consumer disposables. It may take a while for humans or other animals to ingest them, but forever chemicals can last, well, forever. As they build up in and interact with our bodies, they can, according to studies, cause immune problems, birth defects and cancer.

PFAS are exceptionally strong, making these molecules useful for engineering slick surfaces, from non-stick pans to pizza boxes and compostable food containers.
Photo illustration: Stephen Bilenky and Caroline McNiel
As Pica read up on PFAS, she decided she needed to do something about them. So after completing her Ph.D., she joined forces with environmental engineers Jens Blotevogel at CSU and Shaily Mahendra at the University of California, Los Angeles to find ways of removing or destroying PFAS from firefighting foam in water and soil.
The team members wouldn't be able to tell if their treatments worked, however, without knowing which specific PFAS were in their samples in the first place. Two such compounds, called PFOS and PFOA, are particularly well known, but firefighting foams contain many other compounds, both PFAS and otherwise. Understanding these complex mixtures is where the MagLab comes in.
The lab's headquarters houses several special instruments called mass spectrometers. Molecules have different masses, depending on their chemical formulas, and mass spectrometers help scientists identify which chemicals are in their sample by breaking them into fragments and weighing the pieces. But where a scale might use Earth's gravity to measure mass in pounds and ounces, mass spectrometers use powerful electric and magnetic fields to distinguish individual molecules, which weigh in at less than a trillionth of a trillionth of an ounce.
The type of tools at the MagLab, called ion cyclotron resonance (ICR) mass spectrometers, are particularly good at analyzing molecules of very similar mass, since they excel both at resolving the difference between similarly massed compounds and accurately measuring their actual mass. Inside these instruments is a magnet and, the more powerful the magnet, the better the machine's resolving power and mass accuracy.

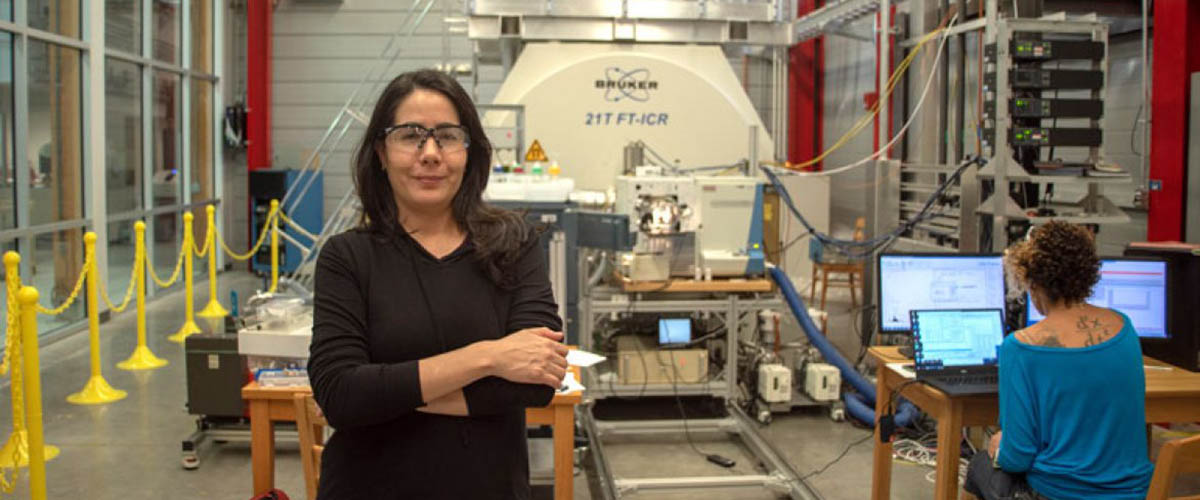
Environmental engineer Nasim Pica (left) and MagLab chemist Huan Chen prepare PFAS samples for measurement in an ICR magnet. The chemicals have been linked to immune problems, birth defects and cancer.
Photo credit: Stephen Bilenky
Working with MagLab scientists Amy McKenna and Huan Chen, Pica measured her samples on a 9.4- tesla x Tesla, or T, is a unit of magnetic field strength; a strong refrigerator magnet is .01 tesla, and a typical MRI machine is 1.5 to 3 tesla. The MagLab's strongest persistent magnet has a field of 45 teslas. spectrometer. The output, like any output of a complicated sample, looks like a jagged mountain range, with peaks representing differently massed fragments of different molecules. Pica's samples are extraordinarily complex mixtures, containing not just PFAS but other additives to the foam and contaminants from the environment. "The first time Amy sent me the list," she recalls, "there were over 20,000 peaks."
Normally this wouldn't be a problem for the ICR machine, which has software to automatically label all of the different peaks in a mixture. After all, the facility routinely analyzes the makeup of petroleum samples containing tens of thousands of different kinds of molecules. But PFAS, explains Chen, "are a newly recognized contaminant, so the detection and characterization methods are not fully developed yet." The instrument's software could match the peaks with many other molecules in Pica's samples, but not PFAS. So she would have to write the software to do that herself.
"I had no coding experience," recalls Pica. "But I really wanted to look at the data, so I had to learn to do it myself." As Pica wrote the software (using an open access tool called UltraMassExplorer) and supplied the samples, Chen and McKenna helped run the experiments and interpret the data. It took about six months, but in the spring of 2018, Pica finally had software that could look at the ICR data and identify which PFAS lie within.
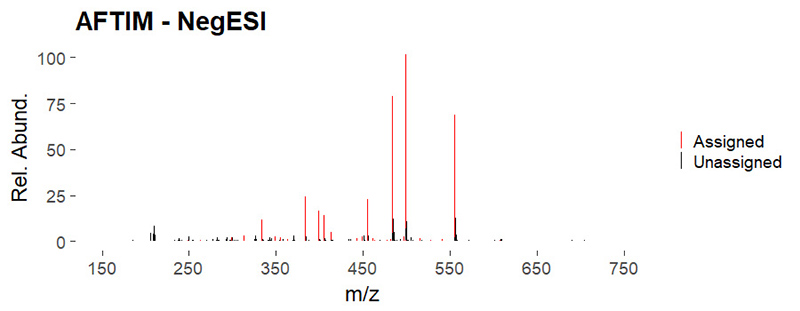
An ICR spectrum from one of Pica’s samples of firefighting foam, which contains PFAS. Each peak of the spectrum identifies a specific molecule in the foam; the molecular formula can be calculated from its measured mass.
Image courtesy of Nasim Pica.
Pica was unsurprised to find many PFAS besides the notorious two — PFOA and PFOS — in her samples. "Industry is moving away from using these two," she says, because of their well-studied danger. However, instead of replacing PFAS entirely, companies are switching to other fluorine-carbon molecules whose effects on health and the environment are still poorly understood.
Identifying which of these compounds filter through the soil into groundwater, and how treatment affects them, is the first step to dealing with them, says Pica, now an environmental engineer with a Wisconsin construction firm. Now that scientists can tell which compounds are in a sample, Blotevogel and his team will be better able to test new techniques for treating water and remediating contaminated soil. Blotevogel, McKenna and Pica are also working on a new project, tracing the origin of PFAS found in the environment, whether from firefighting foam or something else.
Fully solving the problem, though, says Pica, means not only removing PFAS but finding alternatives to using them in the first place. "Yes, PFAS is a problem," she says, "but it's not the problem. The problem is the human demand for a quick answer rather than a sustainable solution."
To learn about other recent PFAS research by Pica and Blotevogel, read, "A solution for cleaning up PFAS, one of the world's most intractable pollutants."
Story by Bennett McIntosh