What did scientists discover?
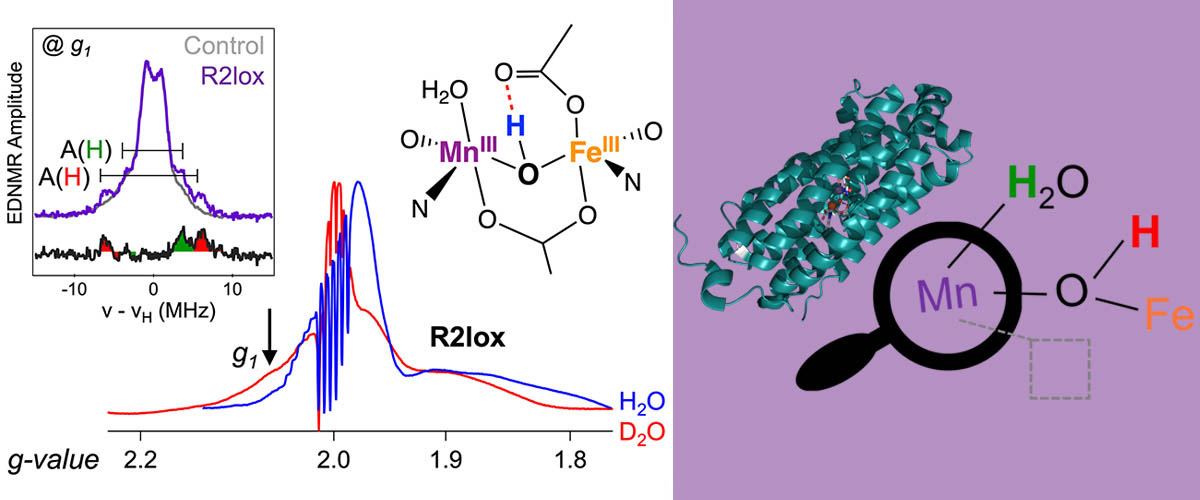
Using high-magnetic-field pulsed electron magnetic resonance, MagLab users were able to interrogate the electrons that are presumed to play an important role in the disease-causing activity of a particular protein in the tuberculosis bacterium, a protein that features an unusual manganese-iron pair of metal atoms. The extremely fine structural information afforded by these experiments revealed an unoccupied atomic site right next to the manganese atom (dashed box in the Figure) which signifies a likely way that target molecules can approach and bind to the metal core. This finding represents a critical first step towards understanding the chemistry that the R2lox protein performs in tuberculosis bacteria.
Why is this important?
Tuberculosis superbugs have evolved many ways to overcome the immune system of their hosts. While all organisms need iron to live, one way that bacteria can survive is to substitute other metals for iron in their metabolisms. In tuberculosis and some other pathogens, the metalloprotein “R2lox” has evolved with a “mixed-metal” manganese-iron (Mn/Fe) site in place of the typical two-iron (Fe/Fe) active site. Because R2lox has been identified as a virulence factor for tuberculosis, understanding its chemistry offers a new and potentially valuable approach towards fighting this disease. This study investigates the structure of the two metals at the center of R2lox during the first reaction cycle with oxygen, with a specific emphasis on the short-lived state that controls downstream reactions.
Who did the research?
E.C. Kisgeropoulos,1 Y.J. Gan,1 S.M. Greer,2 J.M. Hazel,1 and H.S. Shafaat1
1The Ohio State University; 2National High Magnetic Field Laboratory
Why did they need the MagLab?
R2lox shows unusually weak communication between its Mn and Fe atoms, necessitating the ultra-high resolution HiPER instrument available in the MagLab’s EMR user program. This interaction would otherwise be impossible to resolve using commercial low-field spectrometers. Furthermore, in order to study atoms bound to the metals, the uniquely high-power capabilities of HiPER were essential. Indeed, these studies can interrogate both the electrons and protons (hydrogens) involved in the reactivity of R2lox in the same experiment, directly showing which protons interact with the metal center (also shown in the Figure).
Details for scientists
- View or download the expert-level Science Highlight, Probing the Reactivity of a New Class of Metalloproteins With High-Field Pulsed Electron Magnetic Resonance
- Read the full-length publication, Pulsed Multifrequency Electron Paramagnetic Resonance Spectroscopy Reveals Key Branch Points for One- vs Two-Electron Reactivity in Mn/Fe Proteins, in Journal of the American Chemical Society
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779); H.S. Shafaat (NIH GM128852)
For more information, contact Stephen Hill.