Like a lot of commuting parents, chemist Ryan Rodgers drops his kids off at school before heading to work, in his case to the National High Magnetic Field Laboratory. Toting lunches, backpacks and water bottles, his two girls climb into their Tundra and buckle up for the short ride to campus. Behind the family's banter, the group Twenty One Pilots sings a nostalgic ode to a more innocent era.
"Wish we could turn back time, to the good ol' days ..."
At school, Rodgers gives his daughters a pair of high-fives before heading west across Tallahassee to work. It's an easy, 8-mile trip — free of the gridlock many commuters endure. Still, Rodgers knows something most of those drivers don't: Those smooth, asphalt roads that make his morning commute such a breeze may not be so safe after all.

Chemist Ryan Rodgers worries about how asphaltenes, a class of molecule found in petroleum and used in asphalt, are impacting the environment.
Photo credit: Stephen Bilenky
Rodgers has built a career on studying petroleum, the most chemically complex substance on the planet. Using world-leading magnets and instrumentation, he helps oil companies better understand the molecular makeup of the stuff they pump out of the ground and researches the effects of oil spills.
So as Rodgers sips coffee at the wheel of his truck, he understands better than most that he's driving on a petroleum product. Although the road is made mostly of stones, sand and gravel, something needs to glue it all together. That something is a crude oil derivative called asphalt binder (or asphalt cement), a heavy, black, sticky goo made from the bottom-of-the-barrel stuff at the tail end of the distillation process.
By mass, binder makes up only about 5% of a road. But that adds up when you consider the vast surfaces it covers: hundreds of lane miles in Tallahassee alone, 274,000 across Florida, 8.5 million in the U.S. and many millions more crisscrossing the rest of the planet. And all that binder has Rodgers pretty worried.
A few years ago, he and his research group started studying a specific class of molecules found in petroleum called asphaltenes, which are particularly abundant in heavier crude oil distillates. At first, the scientists sought to shed light on the molecules' structure, which they believed had been misunderstood. As their research progressed, they considered how those structures affected the molecules' behavior. Through a series of experiments and publications and with a growing sense of urgency, they have slowly built a case that has not only important scientific significance but also far-reaching economic, environmental and health implications.
In addition to being chemically complex, petroleum is exceptionally useful; we have tapped it to run our cars, heat our homes and create countless consumer goods — from plastic dishware to candles. Given their complexity and ubiquity, it's not surprising that petroleum-based products once thought harmless later turned out to be toxic. From benzene (used in many industrial processes) to naphthalene (found in mothballs) to, according to some studies, BPA-containing plastics and microplastics, some petroleum derivatives can indeed be hazardous to your health.
According to the National Asphalt Pavement Association, the U.S. alone produces about 400 million tons of asphalt pavement material a year, worth more than $30 billion. The work Rodgers and his team have been doing raises serious questions over every last square inch of it.
To understand something as seemingly simple as a paved street, though, requires a detour toward the anything-but-simple, and surprisingly controversial, molecular structure of its asphaltene-rich binder. And because this is science, after all, this road features a few twists, turns and delays as researchers follow their way through the twists and turns of the scientific method.
Questioning accepted wisdom
When scientists first began analyzing asphaltenes in the 1960s, the consensus was that they were made up of a central core of carbon atoms — petroleum coke — with the occasional hydrocarbon side chain (made up of hydrogen and carbon) poking out. When heated, those chains crack off, leaving behind an extremely stable, insoluble core that is not terribly useful. In fact, asphaltenes are often the bane of refineries, where they tend to clump together and clog pipes.
According to this so-called "island" molecular model, asphaltenes are a poor source of fuel, offering no energy-rich molecular bonds to break. Due to their high boiling point, they were also thought to have a high molecular weight. Indeed, measurements from that era suggested that crude oil contained some relatively light molecules (gasoline, for example) and some heavy ones (asphaltenes), but few species in between. That data supported the theory that asphaltenes were mostly heavy clumps of interconnected carbon rings.
By the 1980s, however, some scientists began to doubt this model. These skeptics included Canadian scientists studying heavy crude oil samples from northern Alberta known as Athabasca bitumen, or oil sands. Although these samples were known to contain a relatively high percentage of asphaltenes, they were yielding more fuel than the island model would predict for an asphaltene-rich crude.
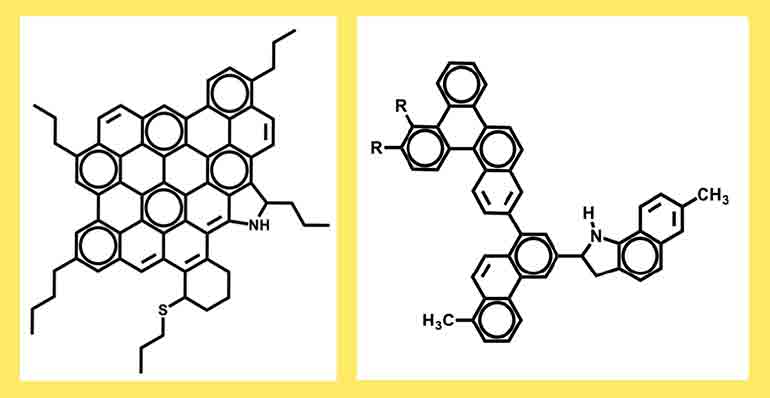
Models of an "island" asphaltene (left) and an "archipelago" asphaltene (right).
Photo credit: Martha Chacón-Patiño
The study of asphaltenes was becoming increasingly relevant because sources of easy-to-refine "light" crude oil, which flows easily and contains relatively few asphaltenes, were drying up. More and more, petroleum companies were left with the "heavier," viscous crudes that contained higher percentages of asphaltenes.
Some industry scientists were also questioning the island model, said Murray Gray, a professor emeritus of chemical engineering at the University of Alberta who has worked with Canadian oil refineries. Their first-hand experience successfully refining heavier crudes suggested that the scientists focusing strictly on experimental measurements were missing something.
"When chemical engineers look at a refinery or processing plant, we are keenly aware that mass in has to equal mass out," Gray said. "So, the chemical engineering experience was that you put the bitumen material containing lots of asphaltenes into processing plants and you got a range of material coming out. That just did not fit at all with this very simple picture of asphaltenes ... it was clear that the actual structures of the asphaltenes were much more complicated and diverse."
Rodgers described it more bluntly: "The refiners were sitting there saying, 'The academics are idiots.'" It's the type of brusque remark his colleagues know to expect from him. A burly, bearded man with no use for gratuitous smiles, Rodgers has a determined gait and piercing blue eyes that make you step out of his path in the hallway.
Eventually, another model for what asphaltenes might look like emerged, one that could be reconciled with the observation that at least some asphaltenes are a good source of fuel. It was dubbed the "archipelago model." Rather than featuring a single large coke core, this hypothesized molecule was made up of several smaller cores linked by side chains — chains that could break and yield energy.
Proponents of this model included chemist Mieczyslaw Boduszynski of Chevron Energy Technology Company, who challenged the idea that petroleum molecules could only be either "light" or "heavy." Rather, he argued that their masses spanned the entire continuum in between, and molecules that boil off at higher temperatures in the distillation process aren't all heavy. The higher boiling point constituents, he proposed, also tend to contain more oxygen, sulfur and nitrogen. These atoms promote stronger associations between molecules, resulting in the higher boiling point. At the time, the technology did not yet exist to confirm his ideas. But if they were true, they would explain how asphaltenes might include not just island structures, but lighter-weight archipelago species as well.
Still, some scientists held fast to the island-only view. After all, it aligned more closely with both the computational models of the time as well as with the data coming out of the instruments used to analyze complex mixtures.
Instrumentation, however, was advancing, and Rodgers was right in the thick of it. In the 1990s, under National MagLab chemist Alan Marshall, Rodgers began building an ion cyclotron resonance (ICR) mass spectrometer capable of analyzing compounds as complex as petroleum, eventually positioning Marshall's group as leaders in the nascent field of petroleomics. Mass spectrometers are essentially fancy scales for molecules that use a strong magnet to identify each molecule in a substance by its unique mass. Developing ICR magnets that could decode petroleum samples containing tens of thousands of different kinds of molecules was a formidable feat.
As Rodgers' expertise in petroleum and instrumentation grew, so did his doubts about the island-only view of asphaltenes. In 2016, he hired Martha Chacón-Patiño, a postdoctoral fellow from Colombia who had studied asphaltenes in graduate school. With other members of the ICR team, they set about to rewrite the book on asphaltenes through a series of experiments and articles designed to chip away at the island-only model and expose the true structure and behavior of these complex compounds.
On the surface, Rodgers and Chacón-Patiño are a study in opposites. A petite brunette, she is as polite as Rodgers is gruff, her speech as meticulous as his is freewheeling. When replying in the affirmative, Rodgers blurts a brusque "Yeah," while Chacón-Patiño patiently nods along to her drawn-out "Yeeeessss." Yet they share a certain inscrutability, perhaps due to having to defend their work against skeptics.
The MagLab's effort to fully decode asphaltenes had three critical things going for it. First, an exceptionally powerful and accurate instrumentation for petroleomics research. Second, access to excellent asphaltene samples: one from Wyoming known to be rich in island structures, the other from Alberta believed to be rich in archipelago structures. Last but by no means least, Rodgers said, was the team's expertise, in particular the talents of his postdoc. Boasted Rodgers, "We have Martha."
Chacón-Patiño has been determined to meticulously build the team's case through strict adherence to the scientific method. Some of the accepted wisdom the MagLab team has been debunking, she said, came about, "because people made many mistakes."
Her mission was to find those mistakes and fix them.
Better techniques, more accurate data
The team set out to refine ICR measurement techniques that would help them detect the molecules they felt had long eluded scientists. Experimenting with island-abundant Wyoming asphaltenes and archipelago-abundant Canadian asphaltenes, this is what they figured out:
Lesson #1: Don't ionize all the molecules at once. Before you can weigh a molecule in a mass spectrometer, you need to give it a positive or negative charge (ionize it) so that the magnet can detect it. The MagLab team learned that when you ionize all the molecules from an asphaltene sample at once, the island structures ionize more efficiently than the archipelagos; as a result, the archipelagos remain all but invisible to the magnet.
The solution? Divide and conquer. Chacón-Patiño separated the molecules into batches by solubility, then ionized and measured them separately. "That helped us to reduce the complexity," she explained.
Lesson #2. To understand a picture, you sometimes need to break it. After properly ionizing the molecules and before weighing them with the magnet, Chacón-Patiño zapped them with lasers, mimicking the heat of the refining process and breaking off some of the side chains.
After fragmenting the Wyoming molecules and weighing the results, Chacón-Patiño saw just what she had expected to see: some heavy molecules representing large coke cores survived on one end of the mass spectrum.
By contrast, the Athabasca molecules, when broken down, weighed in along a much wider spectrum of masses, reflecting a variety of smaller coke cores that pointed to an archipelago structure. "It's like a puzzle," marveled Chacón-Patiño as she sketched the contrasting spectra.
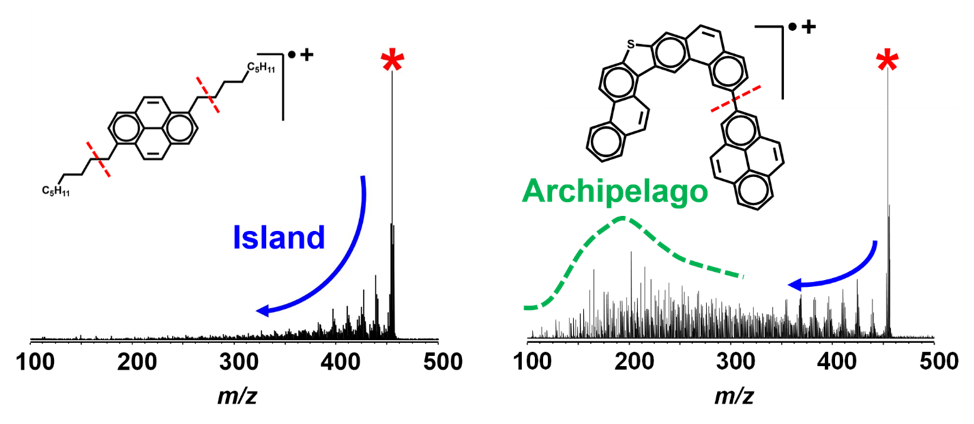
The mass spectra of "island" and "archipelago" asphaltenes are strikingly different.
Photo credit: Martha Chacón-Patiño
Lesson #3: To see wee differences in weight, you need a darn good scale. Molecules are weighed in units called daltons. A water molecule, for example, weighs about 18 daltons. Petroleum is so complex that it contains hundreds of different kinds of molecules just within the span of a single dalton. The high-resolution ICR machines at the National MagLab allow scientists to detect each one of them; less sophisticated instrumentation, Chacón-Patiño said, reveals only a few dozen. So if you use the wrong machine, she said, "You will have the wrong answer."
Up against decades of conventional wisdom, prevailing paradigms and entrenched interests, the MagLab group plotted a strategy: Conduct meticulous research, write up the findings, then lay it all out, one publication at a time. In 2017 they published the first in a series of articles called, "Advances in Asphaltene Petroleomics." Since then three more have followed, and the final paper in the series is due out this year.
Gray was among the academics who quickly embraced the archipelago model, calling the MagLab work "transformative."
"I regard that paper as a major breakthrough because, for the first time, it started to show the full range of chemical structure, demonstrating what we knew all along," said Gray. "What Ryan and Martha are showing is really how you can be misled by artifacts and incomplete analysis."
Other scientists also took notice, and more research on the topic began appearing in the literature. Scientific talks, including one at a symposium honoring Chevron's Boduszynski at the 2017 American Chemical Society's national meeting, generated more buzz. One of Rodgers' prized professional mementoes, in fact, is a 1994 textbook on petroleum by Boduszynski, signed by the author. The inscription reads, "Thanks for proving me right."
It was the scientific method writ large: As knowledge grows and better tools become available, hypotheses are refined, revised and sometimes upended altogether.
Although support for the two-model view continues to grow, the scientific community's response remains "mixed," Gray said. Some may see the archipelago model as a threat to a whole life's work, he said, when in fact many of the advances in the field remain just as valid in a world with two kinds of asphaltenes. "It's going to take time to shift people's belief," he said.
For Rodgers and his team, the case is clearly closed. Their focus now is investigating real-world implications of their research, because a molecule's structure influences its properties. That work is taking them down an even bumpier, more ominous road.
Cloudy water, clear message
Archipelago asphaltenes include more oxygen, sulfur and nitrogen atoms than are found in island asphaltenes. These heteroatoms, as chemists call them, make the molecules more likely to interact with other asphaltenes and with their environment, a tendency with potentially far-reaching consequences.
To learn more about how they react, the MagLab team conducted a fairly simple experiment designed to mimic an oil spill. Chacón-Patiño and graduate student Sydney Niles filled two beakers with artificial sea water. On one they placed a film of Wyoming crude oil (containing asphaltenes that are mostly island); on the second a film of Athabasca bitumen (with mostly archipelago asphaltenes). Then they exposed them to light to simulate what happens to petroleum when it is spilled into the environment, such as in the Deepwater Horizon oil spill.
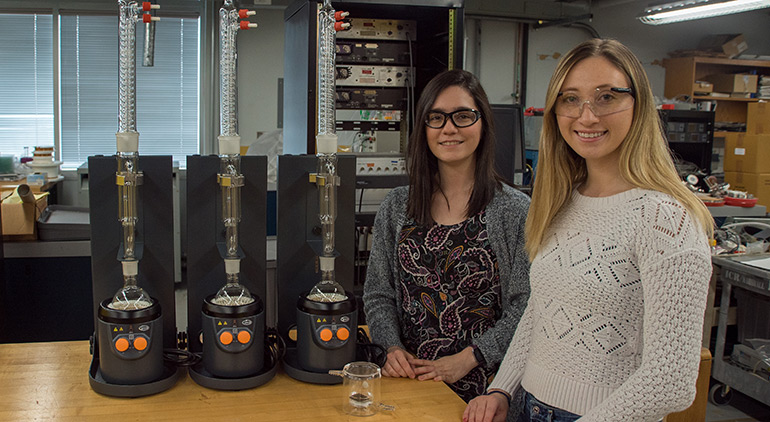
Martha Chacón-Patiño (left) and Sydney Niles in the lab with the Soxhlet extractor they use to prepare samples of asphaltenes for analysis.
Photo credit: Stephen Bilenky
When they checked a day later, one glance confirmed what the complex spectra had previously signaled. The water was no longer crystal clear but, to the scientists, the experimental result was.
In the Wyoming sample beaker, a layer of black, sand-like material had settled at the bottom; it was non-soluble coke that had precipitated out of the island asphaltenes. The water itself was nearly as clear as before the experiment.
In stark contrast, the water in the Athabasca bitumen beaker was murky: Some of the archipelago asphaltenes had dissolved into the water, seemingly contradicting many scientists' long-held belief that asphaltenes couldn't be transformed into water-soluble substances.

Left: The Athabasca bitumen sample after an experiment simulating photooxidation of spilled oil in the ocean. Right: The Wyoming sample after the same experiment.
Photo credit: Martha Chacón-Patiño
So photooxidation caused the island asphaltenes to stick together and form the tar that sank to the bottom; the molecules didn't dissolve. But photooxidation transformed the more reactive archipelago asphaltenes into new species that did dissolve in the water.
The results were exactly what Rodgers expected. Nonetheless, the sight of the dramatically different beakers took him aback. "Science is rarely, rarely that clear," he said.
"That's because we have been so methodical," chimed in Chacón-Patiño, "so we have the results that we have right now."
It's a potent illustration of how one strong image can cut straight to the point. Forget the fancy spectrometer: "You can use your own eyes as the detector," Rodgers said. "I mean — come on!"
Avoiding a road to ruin
The logical next step was one that Rodgers had dreamed of for years: to run the same experiment using samples of asphalt binder. The results were similar to those obtained with the archipelago-heavy crude oil samples from the previous experiment: murky water into which some of the binder had clearly dissolved.
It adds up, said Rodgers. "You see the brand new asphalt roads go down and they're smooth as silk on top. And then you look at one that's 5 years, 10 years old, and you can see the [stone] aggregate. And you're like, 'Where the hell did all the cement go?' And, we now know that it is most likely being photo-converted into water-soluble species. And when it rains, they go out,' into the surrounding environment.
The "they" could be quite a lot of things, some potentially toxic, as certain other petroleum-based products have proven to be. According to studies, soils near paved roads exhibit higher concentrations of polycyclic aromatic hydrocarbons (PAHs), which can be carcinogenic.
"You shine some light on asphalt binder and you get water-soluble species," Rodgers continued. "And not five or six of them: There are tens of thousands of elemental compositions. Each one of those can have [hundreds of thousands of] different structures. So you're just puking ungodly amounts of material that is aromatic x Aromatic molecules are very stable and do not break apart easily. . And it's water soluble, and it has every structure that you can possibly think of. So the idea that one of them is bad for you is probably pretty good."
The results are a vindication of sorts for the researchers, albeit a somber one.
"It's a cool experiment," as Niles put it, "but it's a sad result."
As those results gain acceptance, scientists, industry officials and policy makers will have to face how to deal with them. Could they break linkages between archipelago asphaltenes before using them in binder and other products, and would that even help? Should asphaltenes be banned from certain uses altogether, and is that even feasible?
Rodgers and his team will continue to probe these questions, but finding and implementing answers will almost certainly be passed on to scientists of his daughters' generation. For now, at least, they have the hope that these new methods they've created will advance them much farther down the road toward better answers on asphaltenes.
Story by Kristen Coyne