What is the finding
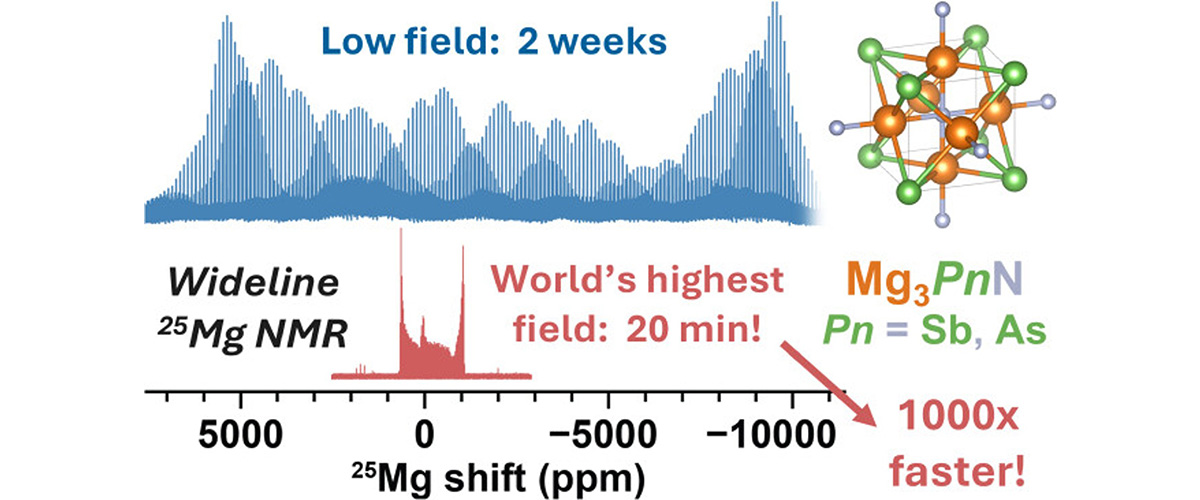
Scientists used the world’s strongest NMR magnet to study how magnesium ions (25Mg) move inside special materials called antiperovskites. The ultra-high magnetic field allowed researchers to perform variable-temperature solid state nuclear magnetic resonance (SSNMR) to measure magnesium’s motion in detail for the first time, finding low-energy pathways.
Why is this important?
Magnesium-ion batteries could store more energy and avoid the safety risks of lithium-ion systems, but progress has been limited because magnesium carries a double positive charge (Mg²⁺) and has difficultly moving through solid materials. Researchers have struggled to identify and validate solid electrolytes that allow fast magnesium transport This research confirms that antiperovskites allow magnesium ions to move easily — a major step toward designing faster, more efficient materials for next-generation batteries critical for energy security and independence.
Who did the research?
David M. Halat1,2,3, Haoyu Liu4, Kwangnam Kim5,6, Grant C. B. Alexander7, Xiaoling Wang8,9, Amrit Venkatesh9, Adam R. Altenhof10, Harris E. Mason11, Saul H. Lapidus12, Jeong Seop Yoon13, Ivan Hung8, Zhehong Gan8, Jordi Cabana7,14, Donald J. Siegel13, Jeffrey A. Reimer2,3, Baris Key4
1Colorado School of Mines; 2University of California Berkeley; 3Lawrence Berkeley National Laboratory; 4Argonne National Laboratory; 5University of Michigan; 6Lawrence Livermore National Laboratory; 7University of Illinois at Chicago; 8National MagLab, Florida State University; 9California State University East Bay; 10Los Alamos National Laboratory (MPA-Q); 11Los Alamos National Laboratory (Chemistry Division); 12Advanced Photon Source, Argonne National Laboratory; 13University of Texas at Austin; 14Argonne National Laboratory (Materials Science Division)
Why did they need the MagLab?
The MagLab’s 36T Series-Connected Hybrid magnet enabled 25Mg solid-state NMR experiments that would be nearly impossible at conventional fields. At 11.7 T, a spectrum must be acquired in small pieces over ~2 weeks. At 35.2 T, the entire spectrum can be captured in only 20 minutes: a time savings of nearly 1000-fold. This unique capability made it feasible to conduct the variable-temperature measurements that revealed magnesium-ion motion in antiperovskites.
Details for scientists
- View or download the expert-level Science Highlight, Probing Mg-ion Transport in Antiperovskites via Ultrahigh Field 25Mg NMR
- Read the full-length publication, Mg-Ion Conduction in Antiperovskite Solid Electrolytes Revealed by 25Mg Ultrahigh Field NMR and First-Principles Calculations, in Journal of the American Chemical Society
Funding
This research was funded by the following grants: D.M.H., J.C., D.J.S., J.A.R., B.K. (DOE BES JCESR, DE-AC02-06CH11357); X.W., A.V., I.H., Z.G. (NSF DMR-2128556, NSF DMR-1039938, NSF DMR-0603042, NIH BTRR 1P41 GM122698); K.K. (DOE DE-AC52-07NA27344); X.W. (DOE DE-SC0025712); D.M.H. (Pines Magnetic Resonance Center Fellowship); J.C., D.J.S., J.A.R., B.K. (DOE Office of Science, Argonne National Laboratory, DE-AC02-06CH11357); D.J.S., J.A.R., B.K. (DOE, Los Alamos National Laboratory, 89233218CNA000001).)
For more information, contact Robert Schurko.