Solid oxide fuel cells are advanced devices that generate clean energy by using hydrogen as fuel and oxygen from the air. They produce electricity without harmful emissions, addressing environmental concerns. These fuel cells don't need recharging and can continuously power things like vehicles and buildings.
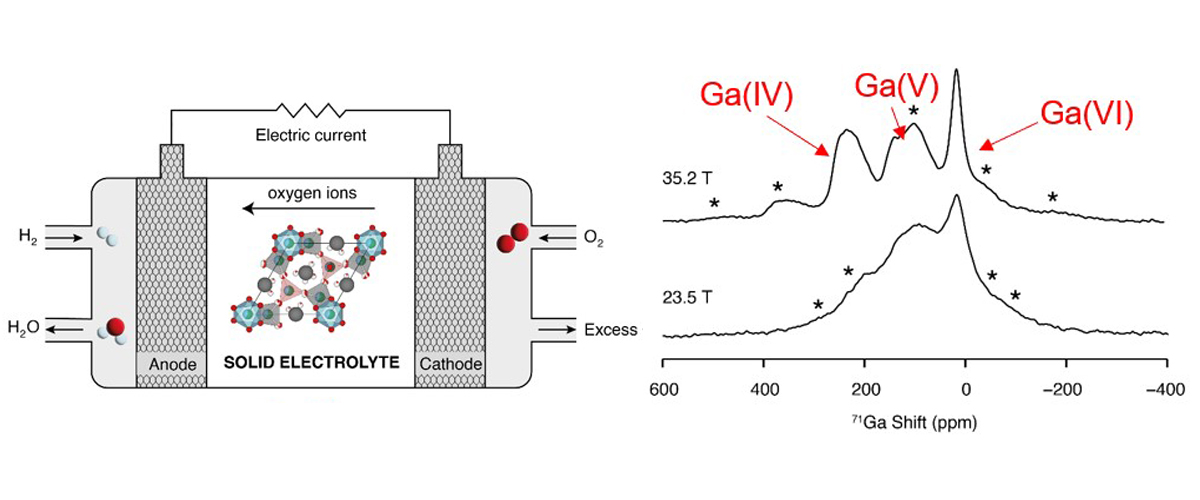
All fuel cells work in a similar way: they have a negative electrode (anode) and a positive electrode (cathode) with an electrolyte in between. In solid oxide fuel cells, the electrolyte moves oxygen ions between the electrodes to convert chemical energy into electricity. This process needs special materials with defects in their atomic structures that can hold different numbers of oxygen ions. Gallium (Ga) is one such element that can do this.
What is the finding?
We developed a method using 71Ga solid-state NMR spectroscopy with the world's highest-field magnet (at the MagLab) to study the number of oxygen ions around each gallium atom in electrolyte materials.
Why is this important?
The function of these materials is controlled by their defect chemistry. Our results clearly show gallium atoms bound to 4, 5, or 6 oxygen ions. This information will help design better electrolyte materials for solid oxide fuel cells.
Who did the research?
Lucia Corti1, Ivan Hung2, Amrit Venkatesh2, Zhehong Gan2, John B. Claridge1, Matthew J. Rosseinsky1, Frédéric Blanc1
1University of Liverpool (UK); 2National High Magnetic Field Laboratory
Why did they need the MagLab?
To see the different gallium sites in the 71Ga solid-state NMR spectrum, we need to use the world's highest magnetic field: the 36 Tesla Series Connected Hybrid magnet. This ultra-high field NMR platform provides a level of detail and spectral resolution that lower fields can’t, allowing us to determine the molecular structures of a wide range of advanced materials.
Details for scientists
- View or download the expert-level Science Highlight, Probing the Chemistry of Fuel Cells with 71Ga NMR Spectroscopy
- Read the full-length publication, Cation Distribution and Anion Transport in the La3Ga5-xGe1+xO14+0.5x Langasite Structure, in Journal of the American Chemical Society
Funding
This research was funded by the following grants: K.M. Amm (NSF DMR-2128556); R.W. Schurko (NIH RM1 GM148766); F.Blanc (Leverhulme Research Centre for Functional Materials Design, EPSRC EP/T015063/1, EP/R029946/1)
For more information, contact Rob Schurko.