What did scientists discover?
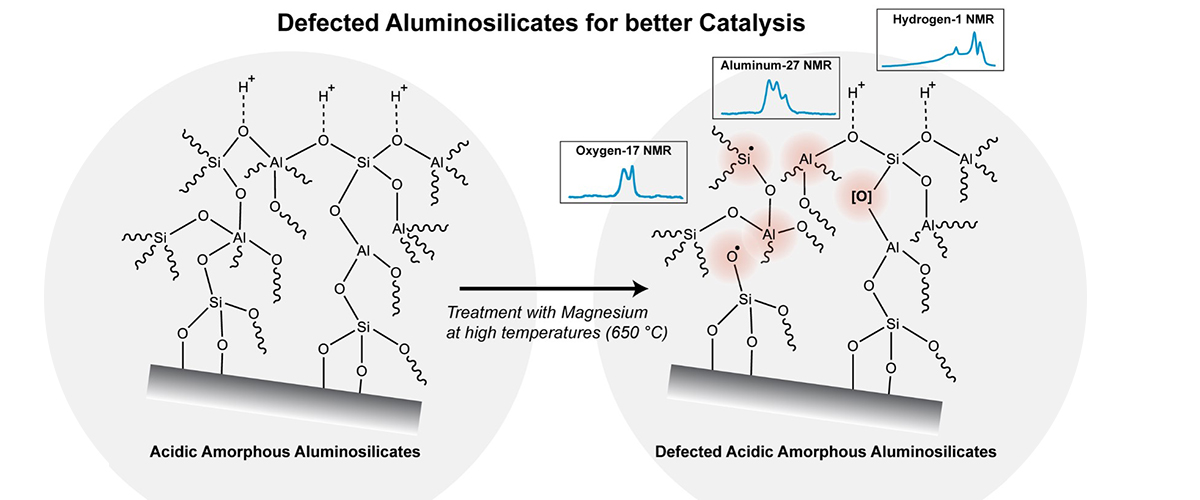
Amorphous aluminosilicates (AAS) are made of aluminum, silicon, and oxygen with disordered, glass-like atomic structures. This work explores how to create tiny "defects" in AAS to make them better at speeding up chemical reactions. By removing certain oxygen atoms through a special process, researchers developed a modified material, D-AAS-12, which proved highly effective in reactions like breaking down styrene oxide and making Vesidryl, a compound used in pharmaceuticals.
Why is this important?
This research shows that creating the right number of defects in materials can speed up chemical reactions by increasing acidity. By improving the acidity of amorphous aluminosilicates, scientists can design better catalysts for specific chemical processes. These advancements could make industrial production more efficient, reduce waste, save energy, and promote more sustainable practices.
Who did the research?
Rishi Verma,1 Charvi Singhvi,1 Amrit Venkatesh,2 and Vivek Polshettiwar1
1Department of Chemical Sciences, Tata Institute of Fundamental Research (TIFR), India; 2National MagLab, FSU
Why did they need the MagLab?
This work needed the MagLab because its powerful high-magnetic field solid-state nuclear magnetic resonance (NMR) instruments can be used to study materials at the atomic level [including the elements aluminum (Al), oxygen (O), and hydrogen(H)]. These special NMR instruments help researchers see a molecular-level view, illuminating how tiny defects in catalysts can impact their structure, acidity, and performance—valuable insights for improving their use in industry!
Details for scientists
- View or download the expert-level Science Highlight, Tuning Acid Strength in Amorphous Aluminosilicates with Oxygen Vacancies
- Read the full-length publication, Defects tune the acidic strength of amorphous aluminosilicates, in Nature Communications
Funding
This research was funded by the following grants: K.M. Amm (NHMFL, NSF/DMR-2128556), V. Polshettiwar (DAE, Government of India, 12-R&D-TFR-RTI4003)
For more information, contact Robert Schurko.