What did scientists discover?
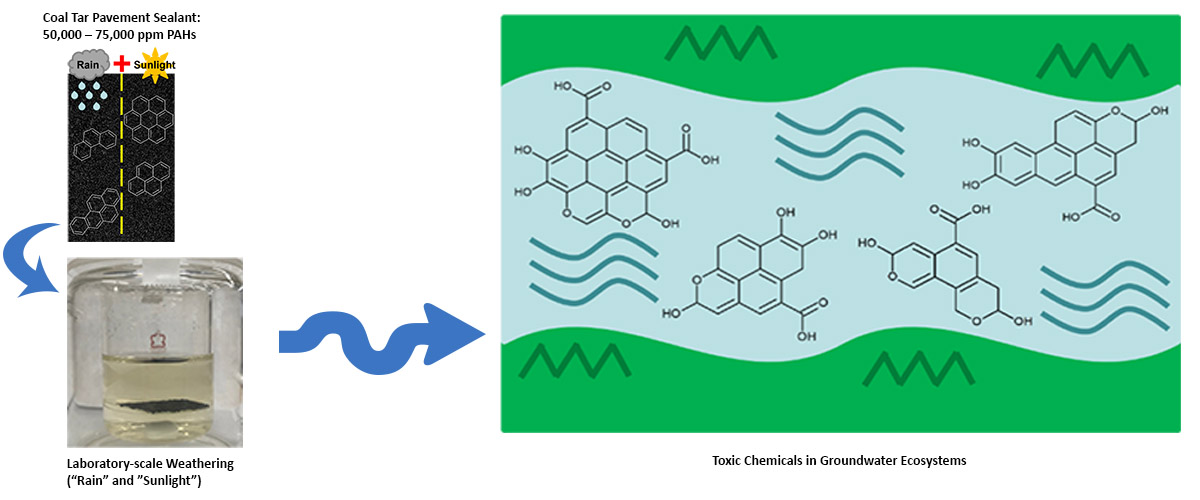
Laboratory simulated weathering of coal tar pavement sealant revealed that carcinogenic polycyclic aromatic hydrocarbons (PAHs) are oxidized by sunlight into oxy-PAHs and then transferred into water. Toxicity testing of water that contained oxy-PAHs revealed high toxicity of water fractions collected from coal tar sealant.
Why is this important?
Little has been known about how pavement sealant is transformed when it is exposed to weathering, such as sunlight and rain. This new research reveals that toxic compounds in coal tar sealant can easily leach into and pollute natural water systems, thus negatively impacting marine ecosystems and public health. These new findings provide critical evidence to support phasing out of these materials in the United States and elsewhere.
Who did the research?
Taylor J. Glattke1,2, Martha L. Chacón-Patiño2, Sarajeen Saima Hoque3, Thomas E. Ennis4, Steven Greason5, Alan G. Marshall1,2, Ryan P. Rodgers1,2
1Ion Cyclotron Resonance Program, National MagLab 2Department of Chemistry and Biochemistry, Florida State University 3Department of Civil & Environmental Engineering, FAMU-FSU College of Engineering 4Watershed Protection Department, City of Austin, TX 5Sitelab Corporation
Why did they need the MagLab?
The MagLab's ultrahigh-resolution FT-ICR (Fourier Transform - Ion Cyclotron Resonance) mass spectrometers allow for identification of tens of thousands of compounds from complex mixtures, such as the weathered coal tar sealant and water samples analyzed in this work.
Details for scientists
- View or download the expert-level Science Highlight, Pavement Sealant Leaches Environmental Contaminants
- Read the full-length publication Complex Mixture Analysis of Emerging Contaminants Generated from Coal Tar- and Petroleum-Derived Pavement Sealants: Molecular Compositions and Correlations with Toxicity Revealed by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry, in Environmental Science and Technology
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779)
For more information, contact Chris Hendrickson.