What did scientists discover?
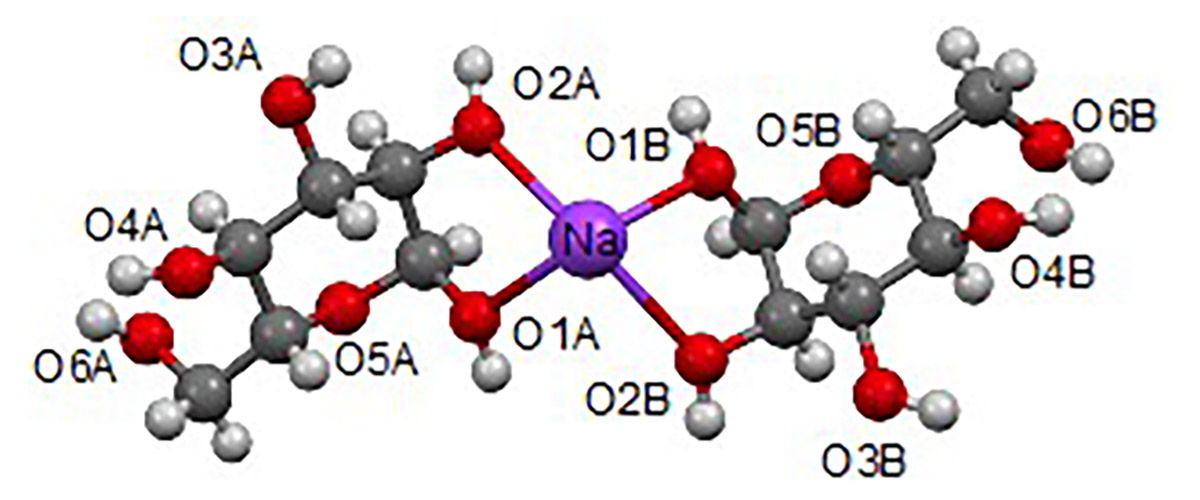
MagLab users have developed a synthetic strategy for introducing 17O-labels into the D-glucose molecule in a site-specific fashion and then applied state-of-the-art nuclear magnetic resonance (NMR) techniques to obtain a complete set of 17O NMR parameters. This is the first time that a complete set of 17O NMR parameters has been recorded for any carbohydrate compound.
Why is this important?
Organic and biological molecules can be thought of as jigsaw puzzles made out of four types of pieces (Hydrogen, Carbon, Nitrogen, and Oxygen). Since 17O is an extremely challenging nucleus to observe, oxygen is the only key constituent of organic and biological molecules that has remained largely “invisible” to the NMR spectroscopic techniques of chemists. So, essentially chemists are trying to solve these puzzles without the oxygen atoms! Here, researchers are working to make these oxygen atoms “visible” to NMR, providing that “last piece of the puzzle” in biomolecular NMR spectroscopy.
Researchers can now explore whether and how 17O-labeled glucose can be used as a new tracer for probing the binding of glucose to other biological macromolecules - such as glucose transport proteins - or for monitoring glycolysis of live cancer cells. It may eventually be possible to extend the same methodology to introduce 17O-labels into more complex carbohydrate molecules such as polysaccharides, or into the ribose/deoxyribose part of RNA/DNA molecules.
Broadly stated, this work adds 17O NMR to the “NMR tool box” available to chemists for the study of the wide variety of oxygen-bearing materials of interest, including organic and biological molecules.
Who did the research?
Jiahui Shen1, Victor Terskikh2, Jochem Struppe3, Alia Hassan3, Martine Monette3, Ivan Hung4, Zhehong Gan4, Andreas Brinkmann2, Gang Wu1
1Queen’s University; 2National Research Council Canada; 3Bruker BioSpin; 4National MagLab.
Why did they need the MagLab?
The measurement required the most powerful NMR magnet in the world, the MagLab’s 35.2T Series Connected Hybrid magnet to overcome the intrinsically low NMR sensitivity of the 17O nuclei. The significantly-boosted sensitivity was critical to the successful detection of 17O NMR signals from D-glucose. The ultrahigh magnetic field also provides an unprecedented NMR resolving power that is uniquely suited to the study of carbohydrate compounds in which all oxygen-containing functional groups are chemically similar.
Details for scientists
- View or download the expert-level Science Highlight, Adding Solid-State 17O NMR to the Tool Box for Studies of Organic and Biological Molecules
- Read the full-length publication, Solid-state 17O NMR study of alpha-D-glucose: exploring new frontiers in isotopic labeling, sensitivity enhancement, and NMR crystallography, in Chemical Science
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779; NSF DMR-2128556); G. Wu (NSERC of Canada RGPIN-2021-03140)
For more information, contact Robert Schurko.