What did scientists discover?
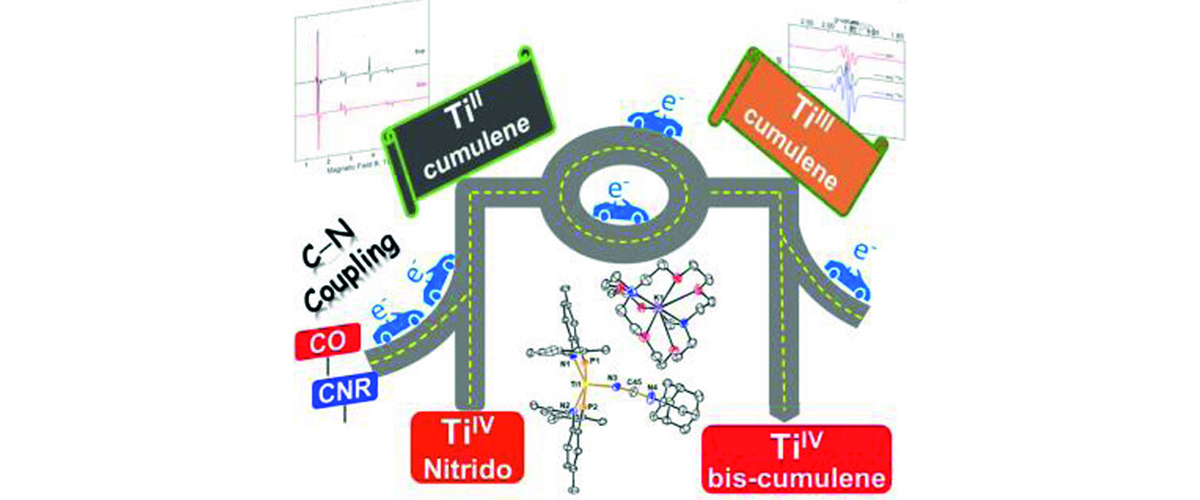
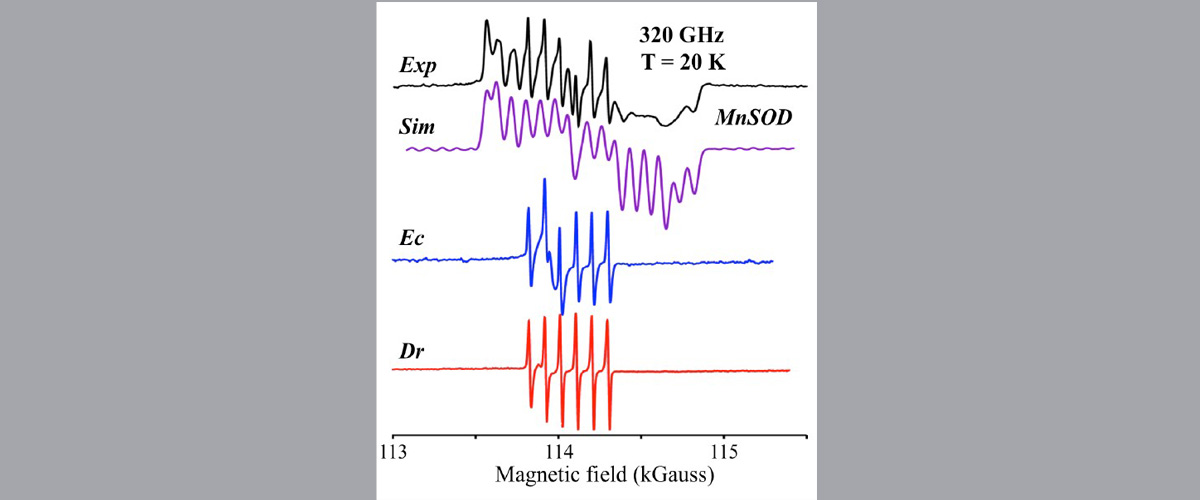
Researchers performed high-field electron paramagnetic resonance measurements on titanium-based catalyst molecules at the MagLab EMR facility and found small, but distinct differences indicating subtle variations in electronic structure. Changes in electronic structure can relate to effectiveness in producing polymers like plastics.
Why is this important?
Titanium is a more abundant transition metal that is less costly than precious metals like palladium (Pd) and platinum (Pt). Titanium-containing molecules are widely used as catalysts for polymers like polyethylene, polycarbonates and other “plastics” and is seen as a promising material for greener, cheaper, and safer catalysts. Understanding how small changes in the molecular structure may affect catalytic performance will help scientists design better catalysts, boosting efficiency and sustainability.
Who did the research?
M. Bhunia1, J. S. Mohar1, C. Sandoval-Pauker2, D. Fehn3, L. N. Grant1, M. R. Gau1, S. Senthil1, J. Goicoechea4, E. S. Yang5, A. Ozarowski6, J. Krzystek6, J. Telser7, B. Pinter2, K. Meyer3, D. J. Mindiola1
1U. Pennsylvania; 2U. Texas El Paso; 3U. Erlangen-Nuremberg, Germany; 4Indiana U.; 5U. Oxford, UK; 6National MagLab; 7Roosevelt U.
Why did they need the MagLab?
Spectroscopic information is essential to understand the electronic structure and functionality of catalysts. Electron paramagnetic resonance (EPR) can provide precise details about the role of unpaired electrons in chemical activity. However, to study transition metals like titanium, very strong magnetic fields like those available at the MagLab are needed because their EPR signals cover a wide range. The MagLab's powerful instruments make these measurements possible.
Details for scientists
- View or download the expert-level Science Highlight, High-Field Electron Paramagnetic Resonance Studies of Titanium-Containing Catalysts
- Read the full-length publication, Softer Is Better for Titanium: Molecular Titanium Arsenido Anions Featuring Ti≡As Bonding and a Terminal Parent Arsinidene, in Journal of the American Chemical Society
- Read the full-length publication, Divalent Titanium via Reductive N-C Coupling of a TiIV Nitrido with Pi-Acids, in Angewandte Chemie International Edition
Funding
This research was funded by the following grants: K. M. Amm (NSF DMR-2128556); D. J. Mindiola (DOE DEFG02-07ER15893); E. S. Yang (UK EPSRC)
For more information, contact Stephen Hill.