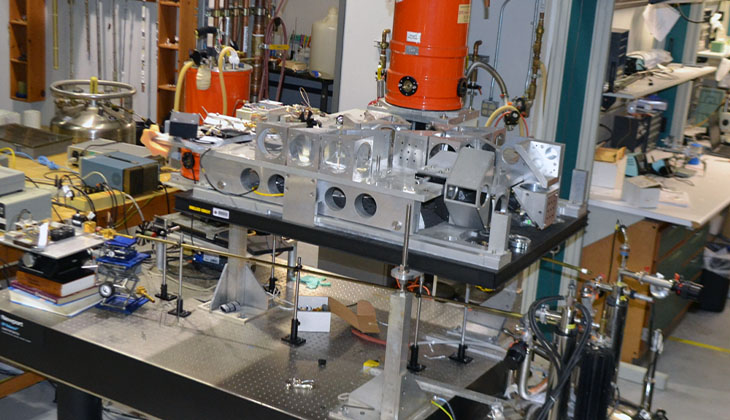
Although the absolute sensitivity of this 24-660 GHz transmission device is lower than that of the quasi-optical system, samples for the transmission apparatus may be very large (0.5 mL and above), thus offsetting the sensitivity disadvantage. The instrument is coupled to a 15/17 T superconducting magnet.
Samples are also substantially larger than in Bruker W-Band, so that spectra are often better than those obtained on the Bruker machine if enough sample is available. Thin Teflon vessels with an outer diameter of up to 9 mm and a depth of up ca. 10 mm are used as sample containers. Standard X-Band 3 mm and 4 mm quartz tubes can also be used for samples requiring sealing. Gelatin capsules up to the size ‘00’ (8 mm diameter) as well as polyethylene vials were also successfully employed. The sample temperature can be controlled over the range of 3K to 309K.
The samples are most often powders or frozen solutions in organic solvents or in water. Single crystals can be measured, but no crystal rotator is available.
The instrument does not employ resonance cavity. The superconducting magnet has been factory-calibrated, but for accurate measurements usage of g standards is recommended (for example DPPH or phosphorus doped into silicon).
Transition metal complexes, some compounds of the f-electron metals (gadolinium) as well as organic radicals have been studied on this spectrometer.
Explore our magnet schedule to see what exciting research is happening on our stellar fleet of instruments right now.
Stoll, S., et al, Hydrogen bonding of tryptophan radicals revealed by EPR at 700 GHz, J. Am. Chem. Soc., 133 (2011) Read online.
Tran, B.L., et al, Reactivity studies of a masked three-coordinate vanadium(II) complex, Angew. Chem. Int. Ed., 49 (2010) Read online.
Krzystek, J., et al, Cobalt(II) “scorpionate” complexes as models for cobalt-substituted zinc enzymes: Electronic structure investigation by high-frequency and -field Electron Paramagnetic Resonance spectroscopy, J. Am. Chem. Soc., 132 (2010) Read online.
Ozarowski, A., et al, High-Field EPR and Magnetic Susceptibility Studies on Binuclear and Tetranuclear Copper Trifluoroacetate Complexes. X-ray Structure Determination of Three Tetranuclear Quinoline Adducts of Copper(II) Trifluoroacetate, J. Am. Chem. Soc., 131 (2009) Read online.
Ozarowski, A., et al, High-Frequency and -Field EPR of a Pseudo-octahedral Complex of High-Spin Fe(II): Bis(2,2'-bi-2-thiazoline)bis(isothiocyanato)iron(II), J. Am. Chem. Soc., 126 (2004) Read online.
Last modified on 04 April 2025