In 1985, chemist Harry Kroto and colleagues at Rice University in Houston zapped a pile of graphite with a laser and discovered a strange new molecule. It consisted of 60 carbon atoms arranged in a pattern similar to that of a soccer ball.
They named their new material buckminsterfullerene, after the geodesic domes of visionary architect Buckminster Fuller; others gave it the catchier term "buckyballs."
The discovery inspired an avalanche of research into the molecule's unique mechanical and electrical properties, and netted its discoverers the 1996 Nobel Prize in Chemistry.
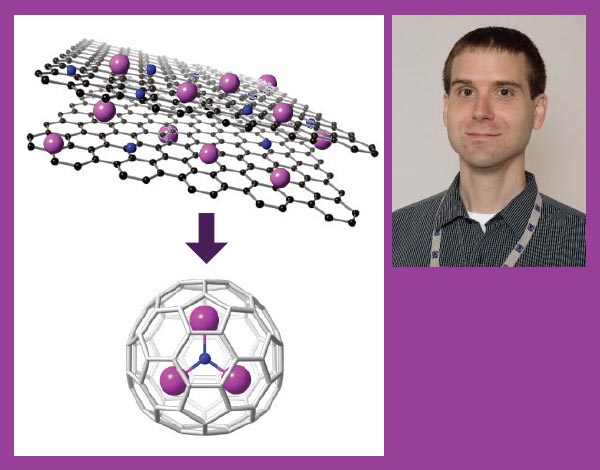
Left: Dunk's research has illuminated how carbon nanocages form when graphite, a metal and nitrogen are vaporized with a laser. (Credit: Paul Dunk); Right: Paul Dunk. (Credit: Stephen Bilenky)
Paul Dunk, a former chemist at the National MagLab in Tallahassee, Florida, who worked with Kroto until his death in 2016, has helped make these properties useful. He's not studying C60, but other strange and wondrous forms that appear when he zaps graphite, metals and other elements with a laser.
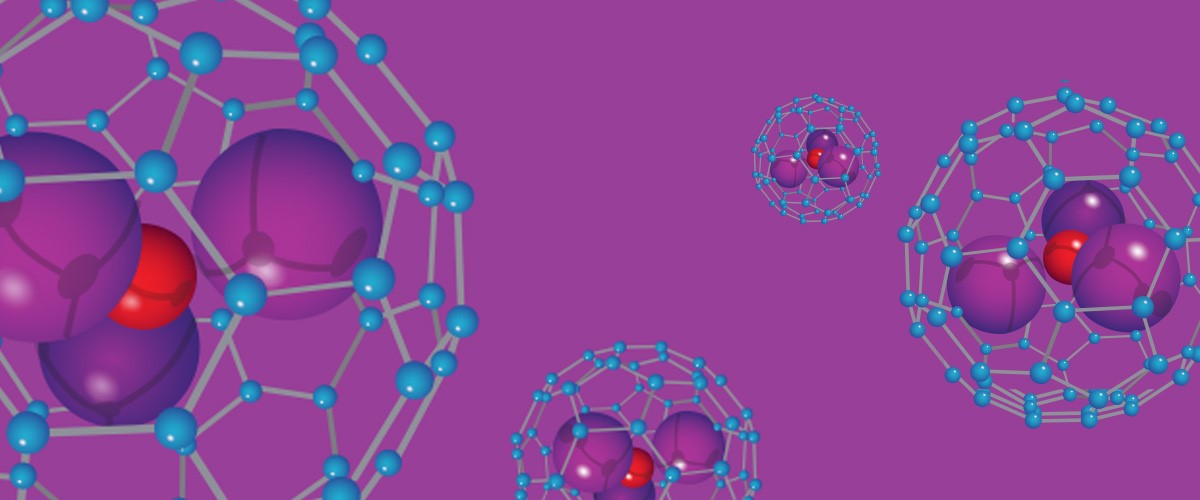
Hints of the field's potential appeared as early as 1999, when scientists unexpectedly found carbon molecules made up of 80 atoms, rather than 60. Like C60, C80 was stable, robust and symmetrical. But these larger buckyballs held a surprise. Inside each molecule's walls were three atoms of a metal called scandium and a nitrogen atom. C80 could, as it turned out, encapsulate other atoms.
The discovery of these "nanocages" opened up the possibility of using buckyballs to transport tiny cargoes. Gadolinium, for example, is an excellent contrast agent for the magnetic resonance imaging (MRI) technology often used in medicine, but it is also toxic. Gadolinium in a nanocage, however, could safely travel through the body to a site where it’s needed.
But there is a holdup: No one understands the violent, chaotic reactions that bring these nanocages into being, much less how to manufacture them consistently and cheaply.
Dunk and collaborators in Texas and Spain are trying to change that. They vaporize concoctions of graphite mixed with different metals and other elements, and study the resulting nanocages in an ion cyclotron resonance mass spectrometer, a device built around a powerful magnet that weighs molecules. In this machine, each molecule circulates at a slightly different frequency that depends on its mass and electric charge. Dunk and his colleagues have used the device to study virtually all the elements in the periodic table to show which metallic atoms can take up residence inside fullerenes.
"The variety is staggering," he said.
The difference between the frequencies in mass spectrometry becomes more pronounced as the magnetic field strength increases, so the National MagLab's world-record instruments help Dunk separate and identify the vast diversity of molecules he produces in the lab.
"With other mass spectrometry methods that don't use high magnetic fields, you can't get that ultra-high resolution, so most of these nanocages have been basically invisible and undetectable," Dunk said. "That high magnetic field allows us to probe many self-assembly processes for the first time, and to develop new nanocages. It fills a really huge gap in nanoscience."
Beyond creating new metallofullerenes, Dunk and his colleagues tested theories of how these compounds form by looking for hypothesized intermediate molecules between the original reactants and end products. They have shown that, unlike what many scientists believed, the cages do not shrink from or break off of larger globs of carbon, but rather nucleate around the metal, carbon atom by carbon atom. A paper they have written describing the process is slated for publication later this year.
With this improved understanding, the researchers hope to pave the way toward more controllable and efficient methods for manufacturing cluster nanocages for technologies ranging from new light-based electronics (or photovoltaics) to molecular electronics.
"There are a lot of new structures that have unique properties that have not been found,” Dunk said. “We hope to accelerate that process."
Added Dunk, "If (cluster nanocages) can be formed in sufficient quantity at the right price, the applications that tackle human health and energy concerns could become an immediate reality."
By Gabriel Popkin
This story was originally part of a series of stories called Going Nano about how good science can come in tiny packages — all with the aim of solving really big problems. To read the other stories in the series, see Magnetism for Miniaturization and Nano Drug Delivery is Rocket Science.