What is the finding
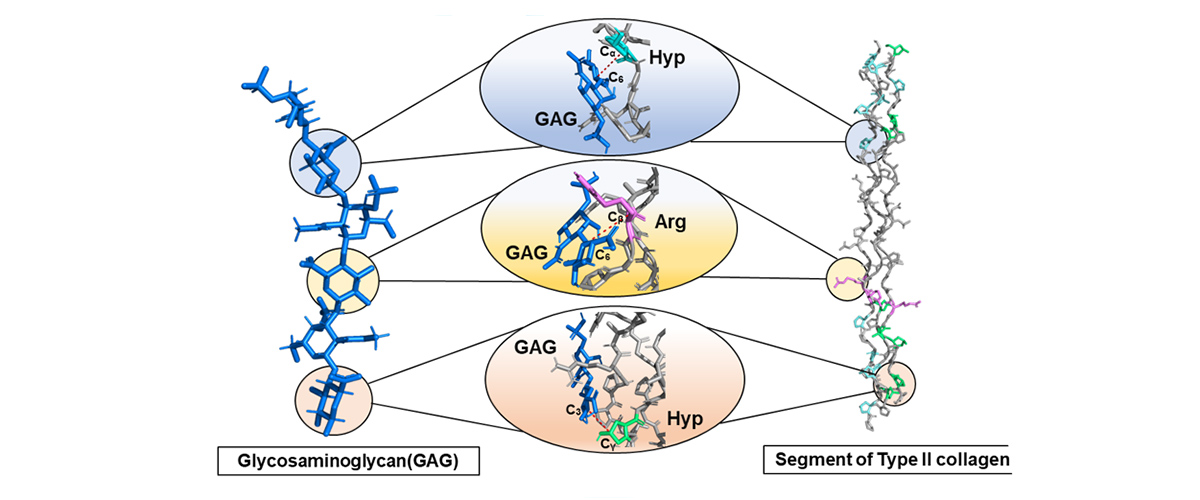
Cartilage is a flexible tissue that cushions joints, supports the ear and nose, and provides a smooth surface for movement while lacking blood vessels and nerves. Scientists discovered that the molecules that make up cartilage — particularly highly mobile glycosaminoglycans GAGs and collagen (a structural protein that provides strength, flexibility, and support to connective tissues) — are held together by specific electrostatic and hydrogen bonding interactions. This is the first time these connections have been directly observed at the atomic level in natural cartilage.
Why is this important?
Studying cartilage at the molecular level helps scientists understand what makes it strong and flexible. This knowledge could help create better treatments for arthritis and improve medical materials for repairing joints.
Who did the research?
Navneet Dwivedi1, Bijaylaxmi Patra1,2, Frederic Mentink-Vigier3,4, Sungsool Wi3,4* and Neeraj Sinha1,2,*
1Centre of Biomedical Research, Lucknow INDIA; 2Academy of Scientific and Innovative Research, INDIA; 3Florida State University, USA; 4National MagLab, USA
Why did they need the MagLab?
The MagLab has one of the world's most powerful NMR instruments with dynamic nuclear polarization (DNP), capable of detecting very weak signals from naturally occurring isotopes in biological tissues. Using a special magic-angle spinning/DNP technique at extremely low temperatures (-279.67°F), researchers greatly enhanced these signals, allowing them to see tiny molecular interactions that other methods miss. This breakthrough helps study biological structures without the need for costly modifications like isotopic labeling.
Details for scientists
- View or download the expert-level Science Highlight, Unveiling Salt-Bridge Interactions Between GAGs and Collagen Protein in Cartilage by DNP-Enhanced ssNMR Spectroscopy
- Read the full-length publication, Unveiling Charge-Pair Salt-Bridge Interaction Between GAGs and Collagen Protein in Cartilage: Atomic Evidence from DNP-Enhanced ssNMR at Natural Isotopic Abundance, in Journal of the American Chemical Society
Funding
This research was funded by the following grants: K.M. Amm (NHMFL, NSF/DMR-2128556 and DMR-1644779), R.W. Schurko (NIH P41 GM122698 and NIH RM1-GM148766)
For more information, contact Rob Schurko.