First, some background
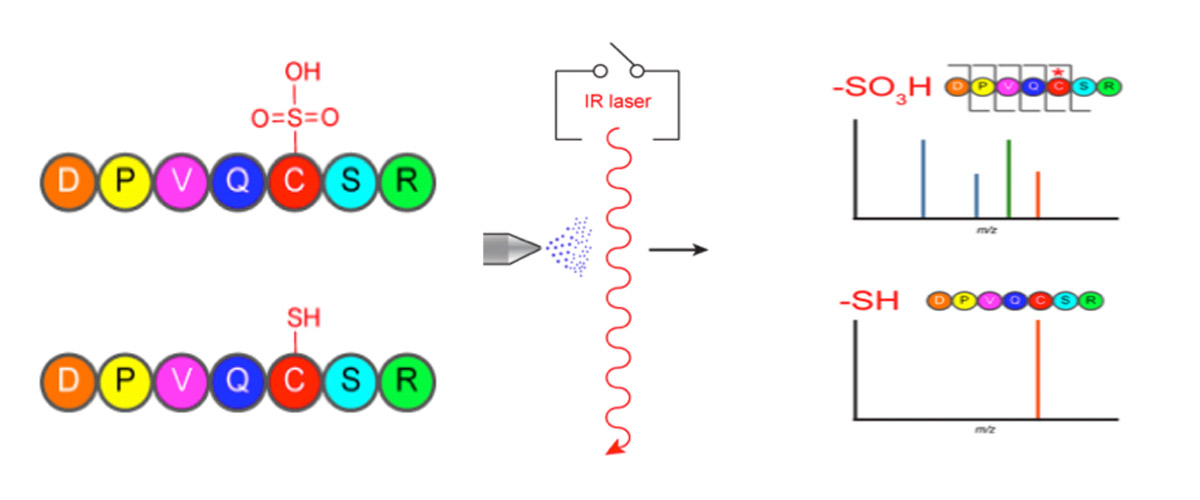
Reactive oxygen species (e.g., hydrogen peroxide) are important second messengers in cellular signaling. In proteins, the amino acid cysteine is particularly susceptible to being oxidized as a post-translational modification (PTM). PTM refers to the modification of a protein following its synthesis by RNA.
What did scientists discover?
High-magnetic-field ion cyclotron resonance (ICR) enabled the development of a new method to rapidly determine oxidative products of sulfur amino acids in proteins by using a CO2 laser to selectively break specific chemical bonds.
Why is this important?
Mis-regulation of the oxidation of cysteine has been linked to hypertension, cancer, aging and neurodegenerative diseases. Other techniques are too insensitive to identify all sulfonic acid-containing peptides following protein oxidation.
Who did the research?
Borotto, N.B.1, McClory, P.J.1, Martin, B.R.1, Hakansson, K.1
1Department of Chemistry, University of Michigan
Why did they need the MagLab?
The selective infrared multiphoton dissociation (IRMPD) technique was able to significantly fragment oxidized peptides to rapidly identify modified peptides. Importantly, IRMPD-generated ions often allowed the localization and assignment of the post-translational modification to a single amino acid residue. The ability to rapidly identify and sequence modified peptides in a single liquid chromatography mass spectrometry (LC/MS) run is a unique capability of the MagLab’s 9.4 T ICR mass spectrometry system.
Details for scientists
- View or download the expert-level Science Highlight, Targeted Annotation of Peptides by Selective Infrared Multiphoton Dissociation Mass Spectrometry
- Read the full-length publication, Targeted Annotation of S-Sulfonylated Peptides by Selective Infrared Multiphoton Dissociation Mass Spectrometry, in Analytical Chemistry
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1157490); Hakansson (NIH R01 GM107148); Martin (NIH DP2 GM114848)
For more information, contact Chris Hendrickson.