
Electron Magnetic Resonance hosts a variety of magnetic resonance techniques associated with the electron.
The EMR Facility provides unique instruments for Electron Magnetic Resonance studies of paramagnetic centers, magnetic molecules, and magnetic materials at very high magnetic fields and frequencies.The most popular of those techniques is Electron Paramagnetic/Spin Resonance (EPR/ESR). In simplified terms, EPR/ESR can be performed on any sample that has unpaired electron spins.
This facility provides researchers with a large range of applications in physics, materials science, chemistry and biology, including studies of impurity states, molecular clusters, antiferromagnetic, ferromagnetic and thin film compounds, natural or induced radicals, optically excited paramagnetic states, electron spin-based quantum information devices, transition-metal based catalysts; and for structural and dynamical studies of metallo-proteins, spin-labeled proteins and other complex bio-molecules and their synthetic models.
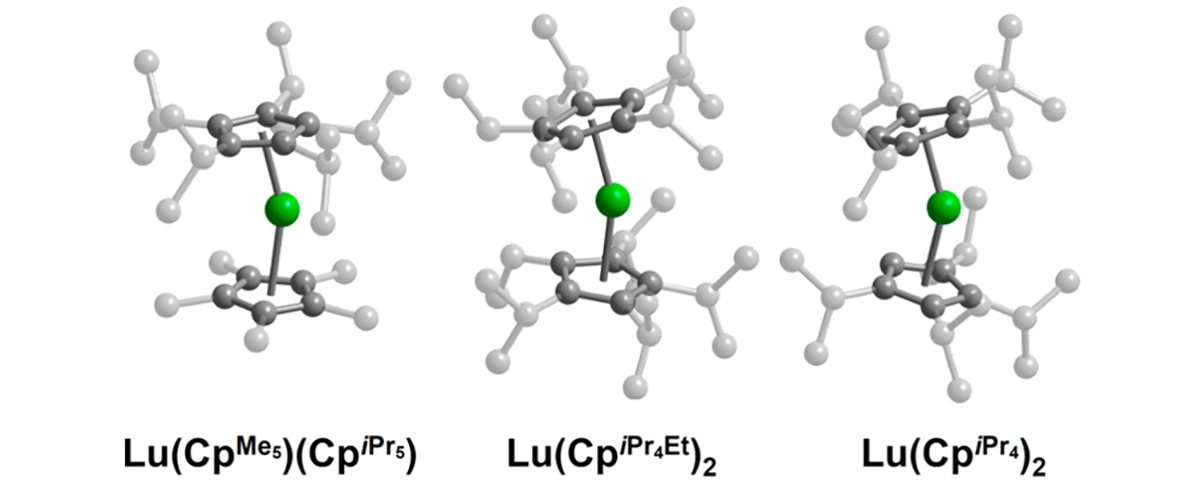
Ngo, D.X.; McClain, K.R.; Hrubý, J.; Franzke, Y.J.; Kundu, K.; Kwon, H.; Gould, C.A.; Harvey, B.G.; Hill, S.; Long, J.R., Journal of the American Chemical Society, 147 (16), 13799-13807 (2025).
Read the full publication https://pubs.acs.org/doi/10.1021/jacs.5c01947 online.
Manvell, A.S.; Dunstan, M.A.; Gracia, D.; Hrubý, J.; Kubus, M.; McPherson, J.N.; Palacios, E.; Weihe, H.; Hill, S.; Schnack, J.; Evangelisti, M.; Pedersen, K.S., Journal of the American Chemical Society, 147 (9), 7597-7603 (2025).
Read the full publication https://pubs.acs.org/doi/10.1021/jacs.4c17003 online.
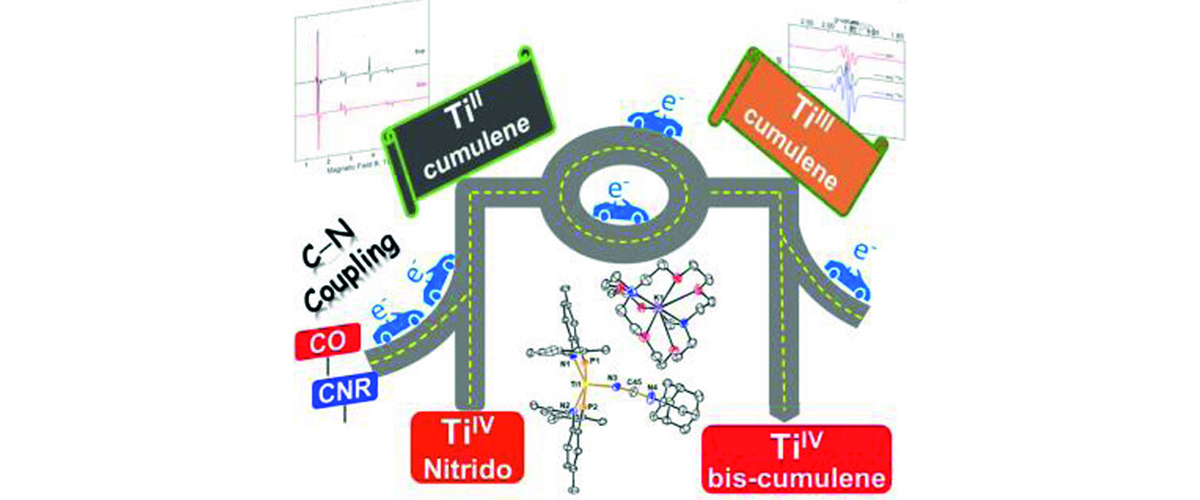
Bhunia, M.; Mohar, J.S.; Sandoval-Pauker, C.; Fehn, D.; Yang, E.S.; Gau, M.; Goicoechea, J.; Ozarowski, A.; Krzystek, J.; Telser, J.; Meyer, K.; Mindiola, D.J., Journal of the American Chemical Society, 146 (6), 3609-3614 (2024)
Read the full publication https://pubs.acs.org/doi/10.1021/jacs.3c12939 online.
Our magnets are open to all scientists - for free - via a competitive process and we accept proposals through out the year.
After your work is completed, you are asked to report to the laboratory, in a timely manner.
Note: There are no deadlines or time windows for EMR proposals.
User Program Administrative Coordinator
For information regarding submitting a proposal and inquiries related to the EMR group