Working in part at the National MagLab, scientists discovered that lanthanum hydride (or LaH10, made up of one lanthanum atom and 10 hydrogen atoms), when under extreme pressure, is superconducting at temperatures as “warm” as -10 degrees Fahrenheit (-23 degrees Celsius). Their findings were published today in the prestigious journal Nature.
Though indeed frigid, that temperature — about the average low for an Alaskan night in January — is far warmer than the previous record for superconductivity, -94 degrees Fahrenheit (-70 degrees Celsius). The achievement puts room-temperature superconductivity (generally pegged at 290 Kelvin — about 17 degrees Celsius or the low 60s in degrees Fahrenheit) within striking distance.
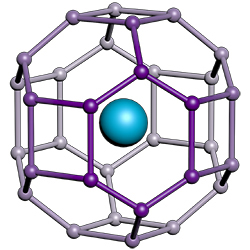
Representation of lanthanum hydride showing a lanthanum atom (blue) surrounded by hydrogen atoms, including those belonging to neighboring molecules in the compound’s atomic lattice.
Image credit: Kevin John
Mikhail Eremets, a physicist at the Max Planck Institute for Chemistry and corresponding author on the study, called the feat a major advance in the competitive, fast-paced world of superconductivity research.
"Even three years ago it was simply unimaginable," Eremets said during a recent visit to the National MagLab, where he continues to research hydrides, the hydrogen-containing compounds that are the latest physics frontier for exploring superconductivity. Eremets was on the team that, in 2015, first discovered a superconducting hydride (hydrogen sulfide, also called sulfur hydride or H3S).
In superconductivity, electrons travel with perfect efficiency: Unlike in normal metals such as copper, there is no loss of energy through heat when the electrons move. The first material discovered to have this property — mercury, in 1911 — could only achieve this state at -452 degrees Fahrenheit (-269 degrees Celsius), greatly limiting its practical applications. Ever since, scientists have been hunting for compounds that possess this capability at ever higher temperatures. A so-called "room-temperature superconductor," it is hoped, would revolutionize the planet by slashing power distribution costs, among other applications.
In Eremets's latest experiments, lanthanum hydride was coaxed to near-room-temperature superconductivity at 170 gigapascals (upwards of 24 million pounds per square inch), a pressure far higher than at the bottom of the ocean. Although such un-Earth-like conditions can only be fabricated using special equipment in a laboratory, the experiments suggest that room-temperature superconductivity could one day be possible under normal atmospheric pressure, said MagLab physicist Luis Balicas, a co-author on the Nature paper.
"There's nothing in nature that prevents you from bringing superconductivity to room temperature," said Balicas, referencing the new study. "That's the conceptual relevance here."
Over the decades, physicists have explored one class of materials after another as they scale the mountain of high-temperature superconductivity. Today, many researchers are competing to reach the 290 K summit first.
The recent entry of hydrides into the century-long race has heated things up for several reasons. First, theorists have played an important role, pointing physicists to specific compounds that, on paper, look like the magic formula. Second, the work of creating these compounds is exceedingly tricky. Finally, the effort overlaps with another great physics quest, the creation of solid metallic hydrogen, theorized to be a superconductor at room temperature.

Eremets and his team conducted experiments on superconducting hydrides created under extremely high pressure using these tiny “diamond anvil cells."
Image credit: Stephen Bilenky
Painstaking process
The series of experiments Eremets and his team undertook were highly challenging. To create the incredibly high pressures needed to form lanthanum hydride, they had to put very small amounts of the raw materials between two miniscule diamonds inside a tiny device called a diamond anvil cell. Then, with the turn of a wee screw, they upped the pressure.
The team conducted a variety of measurements on this delicate sample that called for special instrumentation. MagLab physicist Fedor Balakirev developed a tool that helped control the temperature in the cell while it was subjected to extreme pressure and magnetic fields.
"We go to extra lengths to ensure that our temperatures are very reproducible," said Balakirev, a coauthor on the paper.
To prove the presence and nature of superconductivity in lanthanum hydride, the team subjected the compound to a number of key tests. They measured its electrical resistance in its superconducting state (for superconductors, that value is zero) and confirmed its structure using X-ray diffraction.
The team also sought to confirm the kind of superconducting state they found in LaH10. Scientists have identified two types of superconductivity, conventional and unconventional. Conventional superconductivity, explained six decades ago by the Bardeen Cooper Schrieffer (BCS) theory, is much better understood.
Eremets’s team found evidence that the BCS theory accounted for lanthanum hydride’s superconducting state with two types of measurements. At the National MagLab, they put the compound inside a magnet and subjected it to magnetic fields, which, like other extreme environments such as very low or high temperatures, can affect a material’s properties. The way in which the lanthanum hydride's superconductivity varied as a function of magnetic field strength indicated that it is a conventional superconductor. In addition, at Max Planck, the compound passed the so-called isotope test. In that experiment, scientists substituted the hydrogen in one sample with deuterium, an isotope of hydrogen with a slightly different atomic mass. The temperature at which this modified compound stopped being superconducting (called the critical temperature) was different from that in the original compound.
"If you see that critical temperature changes — usually it decreases — then this is a signature that it is conventional superconductivity," explained MagLab postdoctoral researcher Shirin Mozaffari, another co-author on the paper.
If this all seems very exacting, it should be, said MagLab Chief Scientist Laura Greene.
"This is an extreme claim, so people are asking for extreme proof," said Greene, a physicist who is collaborating on Eremets's current hydride experiments. "There are many things out there that people are accepting that I don't. But this is one that is so solidly put together."
Because LaH10, as a conventional superconductor, is fairly well understood, scientists studying it could make faster progress in making it work under less extreme conditions, explained Eremets.
"Therefore we can make the next step: Go to lower pressures and, ultimately, to ambient pressures," said Eremets. "Because from a basic point of view, there's no limit: Just find a way, technologically, to make the superconductivity at ambient pressure. What we have done is we simply have shown a way how to do it at high pressure."

(From left to right) Physicists Shirin Mozaffari, Fedor Balakirev and Dan Sun, co-authors on the new Nature paper, conduct another experiment with hydride superconductors at the National MagLab.
Image credit: Stephen Bilenky
In pursuit of metallic hydrogen
Eremets calls lanthanum hydride a "superhydride" because of its large number of hydrogen atoms. The more hydrogens you can pack in, he said, the closer you come to a model of pure metallic hydrogen, a material of intense interest to physicists.
In 2017, physicists from Harvard University reported that they had made metallic hydrogen by squeezing the element under extremely high pressure. Other scientists questioned the claim, and it remains unclear if metallic hydrogen was created or not. Either way, scientists are eager to find a reproducible way of making and studying the stuff, which is theorized to be a superconductor at room temperature. That is one goal driving Eremets’s research with LaH10. In a way, he explained, they have created a kind of the metallic hydrogen, except with a little lanthanum mixed in.
"So it's very close to pure hydrogen, so-called metallic hydrogen," Eremets said. "This is a very big goal. Now we have some model of metallic hydrogen. This is even the most important side of this study."
With more experiments and papers on hydrides in the pipeline — an article on hydrogen sulfide with Mozaffari as lead author is slated to appear in Nature Communications later this month — the field will continue to heat up. And scientists will continue to identify the ingredients of superconductivity in a process akin, as Greene and Balicas recently described it, to deciphering a secret recipe.
"You need a little bit of this kind of spin property, of that kind of Kondo property, of this kind of electron-phonon interaction," said Greene, rattling off some of the possible physics behind the behavior. "There are all these mechanisms … That's why it's so difficult to figure out."
"And that's what makes it so tasty for us, too," Balicas chimed in. "We like all these ingredients."
Story by Kristen Coyne