Contact: Frederic Mentink-Vigier
TALLAHASSEE, Fla. — New research involving Michigan State University and the National High Magnetic Field Laboratory details for the first time how a deadly fungus transforms its cell wall to defend against antifungal drugs.
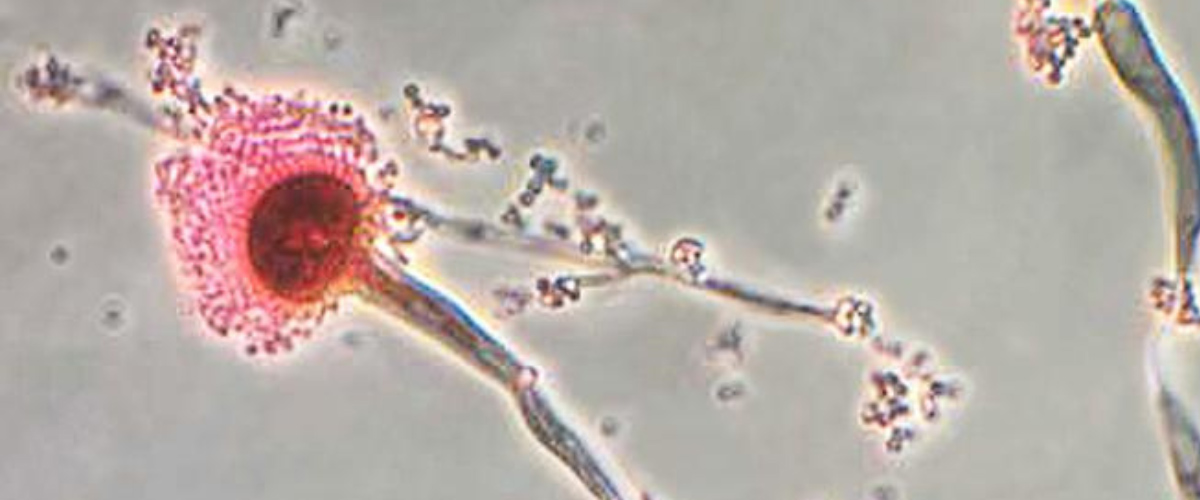
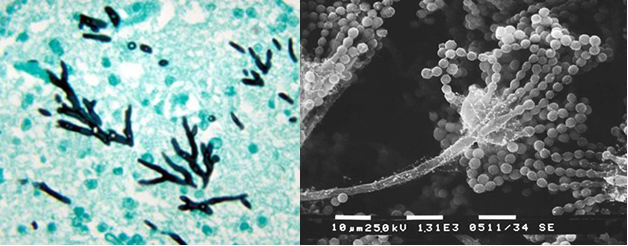
Infections of the fungus, Aspergillus fumigatus, have increased dramatically in recent years. It is now the most prevalent airborne fungal pathogen, according to the National Institutes of Health. Aspergillus fumigatus is found almost everywhere — in our air, soil, and water. People with healthy immune systems are generally not at risk of an infection. But the fungus is a threat for millions who are immunocompromised including COVID patients, transplant recipients, and those with cancer or HIV. The fungus kills an estimated 100-thousand people every year. Even after treatment with antifungal drugs, more than half of patients do not survive an infection.
Centers for Disease Control
The latest class of drugs to fight the fungus, called echinocandins, target the cell walls, blocking production of a key carbohydrate building block. But since their development about 20 years ago, the drugs have become less and less effective.
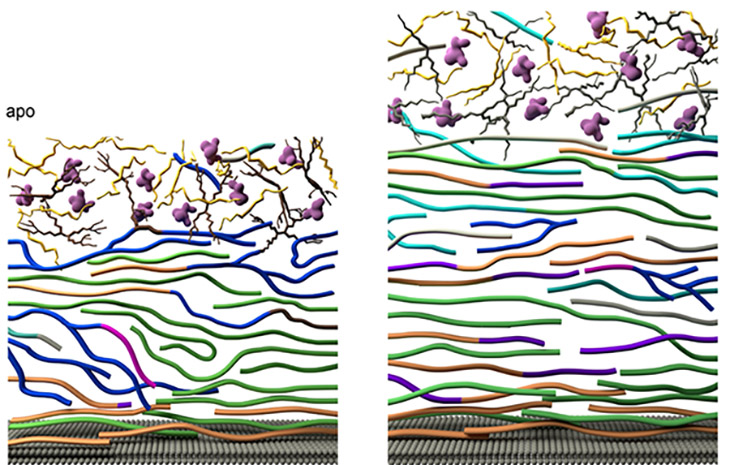
Researchers from Michigan State University, using the MagLab’s world-leading magnet systems, have detailed exactly how the fungi mount a robust defense against the drugs. The team’s findings, published in Nature Communications, show for the first time how Aspergillus fumigatus dramatically modifies its cell wall’s structure. In response to the drug, the fungus reorganizes the large chains of carbohydrates that make up the blocks in the cell wall and shuffles them around.
“It will rebuild its armor completely, reshuffling all the components of the cell wall” said Tuo Wang, the corresponding author of the new study and associate professor in chemistry at Michigan State.
The cell wall thickens and adds new molecules to reinforce itself, the study shows. Longer, rigid molecules called chitin are increased, while less rigid, shorter carbohydrate chains are decreased, and the cell wall becomes more waterproof.
“This dynamic dance unfolds both at the chemical and nanoscale levels, rendering the cell wall sturdier, yet pliable, ensuring survival under stress,” Wang said.
Until now, studies on the fungi’s drug response had only detected a general increase in production of chitin to shore up the cell wall. The MagLab’s world unique Nuclear Magnetic Resonance systems (NMR) allowed for the more detailed analysis. The lab’s 14.1 Tesla magnet with techniques called magic angle spinning and dynamic nuclear polarization harnesses microwave energy and rapid rotation of the sample to improve sensitivity. This system is in part supported by a National Institutes of Health grant which brings cutting edge NMR instrumentation to chemists and biochemists.
"It's not possible to even start this research without this awesome instrument. It is essential," Wang said.
The research also benefited from high-resolution data collected on the 18.8 Tesla NMR system at the MagLab.
Wang, along with MSU graduate students Malitha Dickwella Widanage and Isha Gautam, worked with MagLab faculty researcher Frederic Mentink-Vigier, who says the lab’s equipment offers speed and precision found nowhere else.
"There are so many different molecules coexisting together, and we can map out exactly how they are correlated in space and who is neighbors with who," said Mentink-Vigier. "We understand how the fungi cell wall changes and we understand why it changes."

The study follows previous work by Wang at the MagLab modeling fungal cell walls, detailing the five major types of large carbohydrate chains that make up the wall structure.
The new findings will open avenues for designing new, more effective antifungal treatments that target the altered cell wall. Scientists may also test the effectiveness of combining drugs to attack different cell wall components. And the team plans to expand its research.
"We're looking at the other major fungal species and other antifungal drugs," said Wang. "There are multiple major fungal pathogens, and there are four major classes of antifungal drugs. So there are a lot of combinations and work that we can do."
The MagLab is ready to continue the collaboration and broaden the analysis. Mentink-Vigier says it's gratifying to take part in such cutting-edge science.
"I'm really happy to be able to contribute to this," said Mentink-Vigier. "I'm glad the instrument serves something which has a broad impact. Down the road, this could save lives."
Graduate students Malitha Dickwella Widanage and Isha Gautam were co-first authors of the study.
Data collection was also enabled by the Max T. Roger NMR facility at Michigan State University and Andrew S. Lipton at the Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory in Richland, Washington.
Complementing expertise of modeling and atomic force microscopy was provided by Daipayan Sarkar, Josh Vermaas, and Shi-You Ding, who are colleagues at MSU Plant Research Laboratory, Biochemistry and Molecular Biology, as well as Plant Biology.
This interdisciplinary research also received valuable input from prominent microbiologists, including Thierry Fontaine from Institut Pasteur, Jean-Paul Latge from University of Crete, and Ping Wang from LSU Health Sciences Center, who have provided chemical analysis of cell wall components and advice on adaptive biology of these microbes.