What did scientists discover?
High-magnetic-field pulsed-field-gradient NMR measurements made by MagLab users at our AMRIS facility on catalytic nanoporous gold particles allowed researchers to measure true conversion rates of gas molecules in a porous metallic material for the first time.
Nanoporous gold is a porous metallic material that can catalyze oxidation reactions at room temperature. Taking CO conversion to CO2 as an example for which the mass transport can be straightforwardly studied by pulsed field gradient NMR spectroscopy, MagLab users were able to experimentally assess the impediment created by the pore system present in the nanoporous gold on the transport of gas molecules to the catalytic sites for conversion.
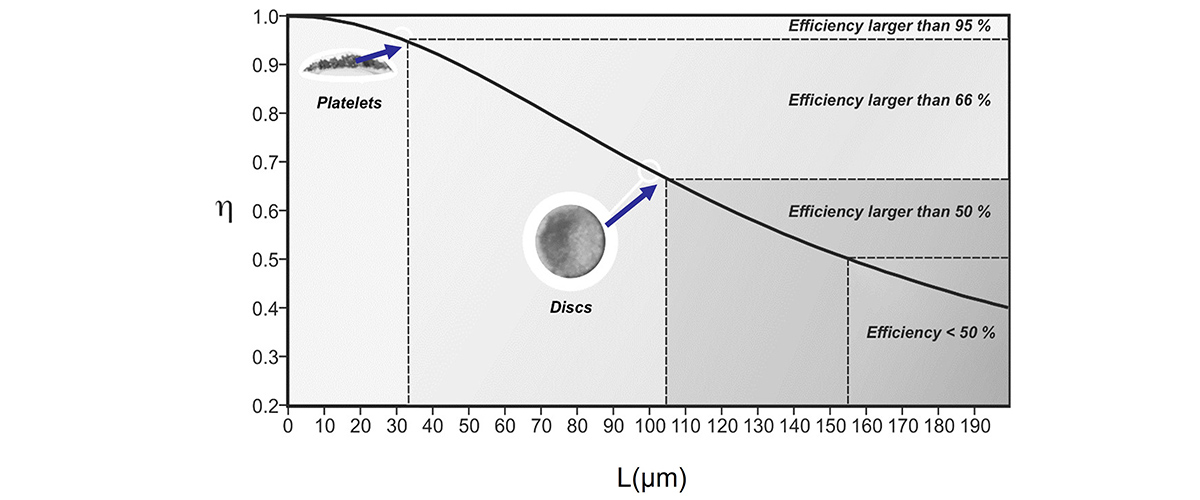
The work included examining differences in gas exchange rates for nanoporous gold particles of different sizes, and determined that smaller platelets were optimal for efficiently catalyzing CO to CO2 oxidation reactions. By breaking up larger (~104µm) nanoporous gold discs into smaller (~33µm) platelets, the reduction of the catalyst lateral size (L) resulted in an increase in catalytic conversion of CO to CO2 by about 50% which, in agreement with predictions, corresponds to an improvement of the effectiveness factor, η, a measure of the normalized reaction rate, to almost 100%.
Why is this important?
The conversion of CO into CO2 is important for cleaning up exhaust gases from power plants and refineries. Knowledge of gas transport rates in nanoporous gold particles allows us to predict more efficient shapes and sizes of a catalyst where transport limitations can be minimized. The improvement of the effectiveness factor shown here may help make the catalyst much more effective in industrial applications, helping to reduce waste and improve efficiency.
Who did the research?
Marcus Bäumer1, Stefan Wild1, Amineh Baniani3, Thomas Risse2, Evan M. Forman3, Sergey Vasenkov3
1University of Bremen; 2Freie Universität Berlin; 3University of Florida;
Why did they need the MagLab?
The high magnetic field realized the higher signal-to-noise necessary to unambiguously identify the optimum particle size for nanoporous gold to catalytically oxidize carbon monoxide to become carbon dioxide.
Details for scientists
- View or download the expert-level Science Highlight, Disentangling Kinetics of CO Oxidation and Mass Transport in Nanoporous Gold using Pulsed Field Gradient NMR
- Read the full-length publication, Disentangling catalysis and mass transport: Using diffusion measurements by pulsed field gradient NMR to reveal the microkinetics of CO oxidation over nanoporous gold, in Journal of Catalysis
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-2128556, DMR-1157490); M. Bäumer and T. Risse (German Research Foundation, BA 1710/29-2 and RI 1025/3-2)
For more information, contact Joanna Long.