Atmospheric pressure chemical ionization (APCI), a widely used ionization technique in mass spectrometry, is similar to atmospheric pressure photoionization (APPI). It is advantageous for its ability to easily ionize and detect nonpolar or slightly polar species.
In APCI, the sample is typically dissolved in a solvent and pumped through a capillary inside an uncharged quartz tube. At the end of the capillary, but still within the tube, the sample is converted into an aerosol and then vaporized with the help of nitrogen gas and by heating to very high temperature (~350-550 °C). The gaseous solvent (S) and sample (M) are then ionized by a corona discharge, in which a highly charged electrode creates an electric field strong enough to ionize nearby molecules. A potential of several kilovolts applied to the electrode typically removes an electron from a neutral molecule without depositing enough internal energy to cause fragmentation. The corona discharge may directly ionize an analyte molecule to form a radical cation (M+●):
M + e- → M+● + 2e-
However, because solvent molecules far outnumber the analyte, it is more likely that a solvent molecule to be ionized in a similar fashion:
S + e- → S+● + 2e-
Frequent collisions between the ions and molecules can transfer charge from an ion to another neutral. Collision of an ionized solvent ion with an analyte molecule can create a direct charge transfer to form a radical cation analyte ion:
S+● + M → M+● + S
Alternatively, collision of solvent ions with a neutral analyte molecule may result in abstraction of a hydrogen atom from the molecule. The resulting ionized solvent can then ionize the analyte via proton transfer:
S+● + S → [S+H]+ + S[-H]
[S+H]+ + M → [M+H]+ + S
The resulting analyte ions (M+● or [M+H]+) are then injected into the mass spectrometer for detection.
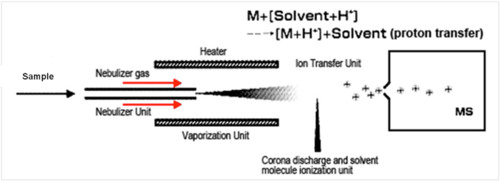
Because ionization occurs in the gas phase, and not the liquid phase, it is unnecessary for the solvent to be polar and able to carry a charge, because no ions need be present in solution. Therefore, unlike electrospray ionization, APCI can ionize nonpolar or slightly polar molecules that do not contain any acidic or basic sites. In addition,, APCI works better at higher flow rates than electrospray ionization, and is thus more easily coupled to a high performance liquid chromatograph. However, in view of the high temperature and need for vaporization, the analyte must be thermally stable and volatile. Highly polar, thermally unstable, or high molecular weight samples, if they vaporize, typically fragment during the ionization process.
Explore our magnet schedule to see what exciting research is happening on our stellar fleet of instruments right now.
For more information please contact Amy McKenna, Manager, ICR User Program.
Last modified on 23 December 2022