What did scientists discover?
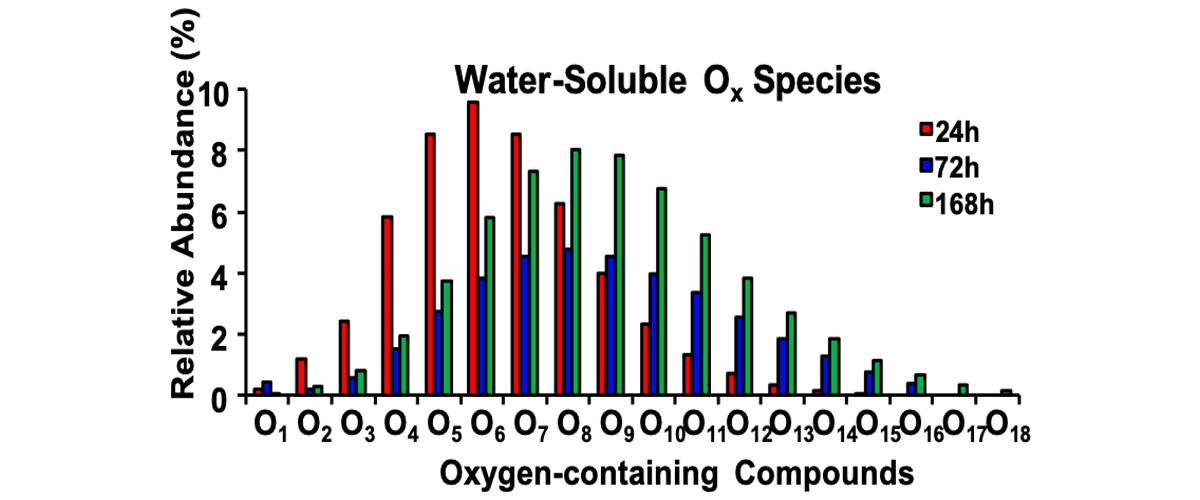
MagLab users found that some chemicals in road asphalt oxidize under simulated sunlight. Compounds with higher oxygen content are more water soluble. As such, these new compounds, created by exposure to sunlight, could leach out of road asphalt during rainfall to cause pollution in the water system.
Why is this important?
Until recently, the tar in asphalt was thought to be essentially chemically inert and therefore environmentally benign. This new research reveals thousands of new, water-soluble chemicals that could be released into the environment by rainfall. The toxicity of the new compounds is being investigated.
Who did the research?
S. F. Niles1, M. L. Chacón-Patiño2, S. P. Putnam3, R. P. Rodgers2, and A. G. Marshall1,2
1Department of Chemistry and Biochemistry, Florida State University; 2Ion Cyclotron Resonance Program, National MagLab; 3Department of Chemistry and Biochemistry, University of South Carolina
Why did they need the MagLab?
The unique ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometers at the MagLab were required to identify the thousands of different chemicals, before and after irradiation. The analysis revealed that irradiation produces abundant new compounds containing many more oxygen atoms ‒ and therefore much higher solubility in water ‒ than the original asphalt. The collaboration has been expanded to include investigators at M.I.T. who will assess the toxicity of the new compounds.
Details for scientists
- View or download the expert-level Science Highlight, Sunlight Produces Water-Soluble Chemicals from Asphalt
- Read the full-length publication, Characterization of an Asphalt Binder and Photoproducts by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Reveals Abundant Water-Soluble Hydrocarbons, in Environmental Science and Technology, Dataset
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1157490, NSF DMR-1644779)
For more information, contact Alan Marshall.