Contact: Kristin Roberts or Dylan Murphy
TALLAHASSEE, Fla. — Scientists doing research at the National MagLab have for the first time characterized the structure of a protein implicated in the onset of some of the most devastating neurodegenerative diseases. Their study, published today on the cover of the prominent biology journal Cell, sheds light on the mechanisms behind these diseases and may guide researchers toward ways to better understand and prevent them.
Diseases like frontal temporal dementia (FTD) and amyotrophic lateral sclerosis (ALS), which together impact tens of millions of people worldwide a year, are still not well understood. However, many scientists believe that, in some of these diseases, so-called “stress granules” form in patient neurons that transition into amyloids — protein fibrils that have hardened into motor neuron-clogging plaque.
Although it’s not well understood how that occurs, the mechanism appears related to a process called liquid-liquid phase separation. This is essentially what happens when you shake a bottle of oil and water: droplets of oil form within, but separate from, the water.
To better understand the formation of protein fibrils, Dylan Murray, a biophysicist at the National Institutes of Health (NIH) and an affiliated scientist at the MagLab, decided to observe it in a test tube. Specifically, he examined a kind of low complexity domain (LCD) protein called the FUS protein, which is known to coalesce into liquid droplets, or a liquid-liquid phase separation, that, over time, mature into something very similar to amyloids.

Dylan Murray
Image: Ulrike Klenke, OITE/NIH
“There’s a parallel between the two, and we’re trying to do experiments in a test tube to learn something about the biology in the cell, which is a much more complicated system” said Murray, who earned a PhD in molecular biophysics at Florida State University under Tim Cross, director of the National MagLab’s FSU-based nuclear magnetic resonance (NMR) program.
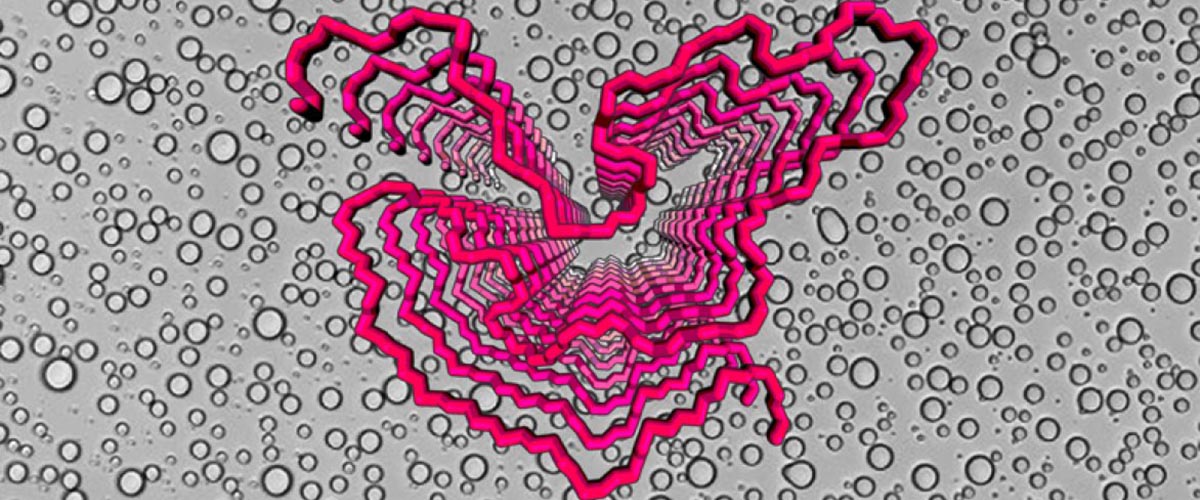
Using NMR instruments at the MagLab and NIH as well as other tools, the research team determined the molecular structure of the FUS protein. Notably, they characterized the fibrils that grow out of protein liquid droplets, which are made of repeating units of protein molecules. After taking precise measurements, the scientists were able to generate a 3D picture of what these fibrils look like.
The team also examined the critical role played by phosphorylation — the process whereby cells attach phosphate molecules to proteins. That action can turn on — or off — certain cell processes. Murray’s research indicated that phosphorylation nips pernicious protein fibrils in the bud.
“We think that phosphorylation of the FUS protein prevents it from forming these amyloid-like structures,” Murray explained. “So, it seems that the cell has the machinery to prevent LCD proteins from forming amyloid-like structures.”
At the same time, the phosphorylation also seems to melt the liquid droplets. The research team even identified the exact amino acids where this beneficial phosphorylation occurs.
A problem arises, however, when that amyloid-fighting “machinery” gets overworked, which may result in neurodegenerative disease.
The Cell study introduces a new way to understand these crippling conditions.
“Until now, nobody has been able to experimentally probe what the structure of the LCD protein is in the droplet form,” Murray explained. “So we were able to provide a glimpse of at least what region of the LCD would be responsible for forming structure in the liquid droplet.”
“This allows us to design future experiments to pin down better what the structures are in the liquid droplet,” he continued, “so that, at the molecular level, we can understand better how they work.”
Murray’s NMR collaborators on the Cell study include his postdoctoral advisor at the NIH, Robert Tycko, NIH staff scientist Kent Thurber, and MagLab staff scientist Ivan Hung. Phosphorylation experiments and segmental isotopic labeling of FUS were done by Masato Kato and Yi Lin in the lab of Steve McKnight at the University of Texas Southwestern Medical Center, as part of a close collaboration with the Tycko lab.
This research was funded by the Intramural Research Program of NIDDK, a PRAT fellowship to Murray from NIGMS (1Fi2GM117604-01), and support to the McKnight lab from NIGMS (5U01GM107623-03).
Text by Kristen Coyne.