Contact: Rongfu Zhang
TALLAHASSEE, Fla. — The global pandemic may be behind us, but there is still a lot we do not know about the COVID-19 virus.
A research team at the National High Magnetic Field Laboratory, in a paper recently published in Communications Biology, is stirring new debate about the structure of a key virus protein.
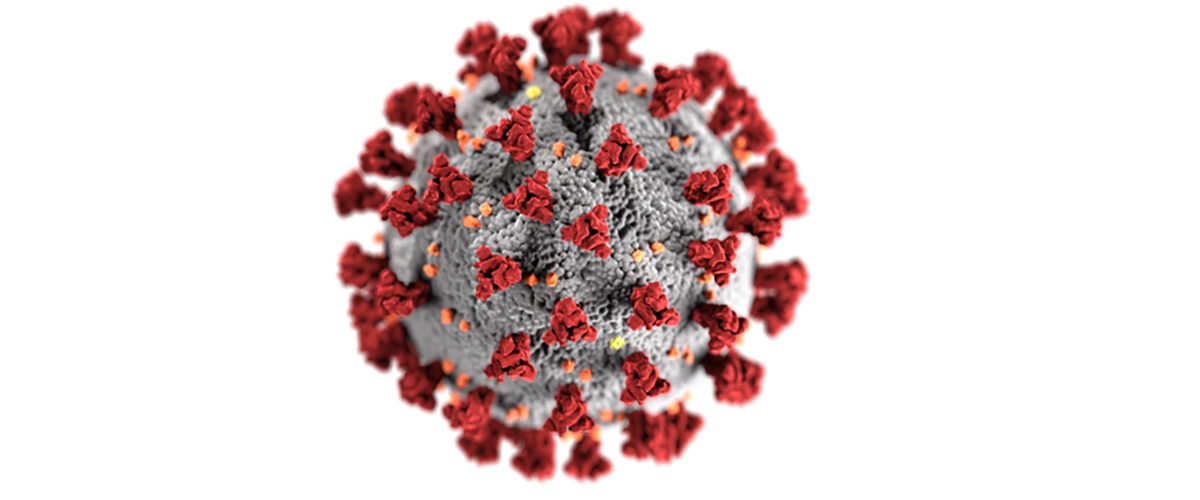
We know that four proteins form the structure of the COVID-19 virus.
Think of the virus as a ball.
The nucleocapsid protein, or N protein, is inside the ball and encapsulates the virus’ genetic material.
The membrane protein, or M protein, dots much of the ball’s surface.
The spike protein, or S, further decorates the outside of the ball with its large bulbous bumps.
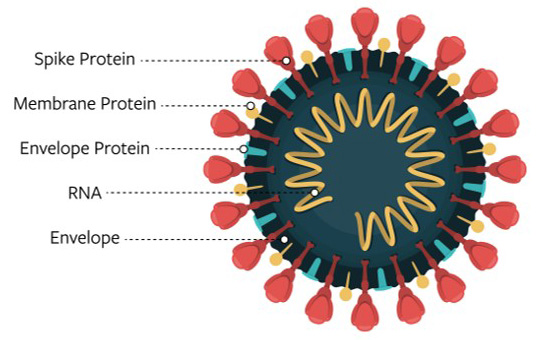
The proteins that form the structure of the COVID-19 virus
Image Credit: National Institutes of Health
MagLab researchers are working to characterize the virus' envelope protein, or E protein. The E protein runs through the viral membrane, or through the surface of the ball in our analogy, and is believed to be key to virus activity.
"We think E protein plays roles when the virus infects human cells, including viral assembly and budding, as well as human cell's immune response," said postdoctoral associate Rongfu Zhang, lead author on the publication.
Previous studies suggest E proteins can organize into a pentamer, which is made of five helices arranged in a pentagon, forming a channel to potentially transport molecules through the virus membrane.
The MagLab group was surprised to uncover quite different results when it analyzed the E protein using solid-state nuclear magnetic resonance. Their work found the protein to be made of just two helices, eliminating any transport channel or pore.
"Our evidence is that it's not a channel," said Tim Cross, FSU chemistry emeritus professor, retired director of the MagLab's NMR User Program, and a paper co-author. "A pore needs to have some sort of a cage. And you can build such a structure with five protein helices, but if you only have two, there's no cavity."
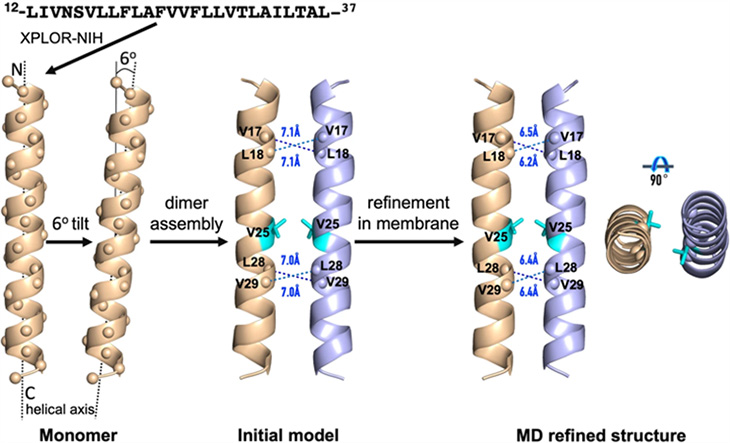
Computer simulation of the two-helix structure of the COVID-19 envelope protein characterized by MagLab researchers.
There are several possible explanations for the conflicting findings.
The previous research used different techniques, which may have provided a distorted structural picture. The MagLab's solid-state nuclear magnetic resonance instruments are some of the best in the world for providing high resolution and accuracy to a team with years of work in this area.
"This group has extensive experience working on this subject. They've been working on transmembrane proteins for many, many years," said Dr. Riqiang Fu, a MagLab faculty researcher and paper co-author. "We have the expertise, the probe development, signal detection, and a fleet of magnets to choose from."
The E protein was also analyzed in slightly different environments.
"We tried to generate an environment that's similar to at least one of the native environments of the protein," explained Cross. "We're trying to model a membrane environment using lipids and lipid bilayers that compose the membranes in our cells."
Researchers speculate that the E protein may take on different forms depending on its environment. For instance, perhaps the protein forms a five-sided channel when it is in great abundance during viral formation but takes on this two-helical form characterized by the MagLab team in a mature virus. The E protein structure could potentially be modified through interactions with the virus' other structural proteins.
"If it's not forming a channel, it may function differently and interact with other proteins over the extensive surface of the virus," said Zhang.
Following these unexpected results, the MagLab group is pursuing more research analyzing the COVID-19 E protein to better understand its structure and function, experiments that could help lead to new anti-viral drugs or COVID-19 vaccines.
"There's always a lot to learn," said Cross. "The problem is the more you learn the more you don't know."
The research was conducted in collaboration with the Department of Chemistry and Biochemistry and the Institute of Molecular Biophysics at Florida State University, along with the Department of Chemistry and Department of Physics at University of Illinois Chicago.
Other authors on the paper include Huajun Qin at Florida State University and Ramesh Prasad and Huan-Xiang Zhou at University of Illinois Chicago.