Electrons, which are like tiny magnets, are the targets of EMR researchers.
EMR stands for electron magnetic resonance. EMR is very similar to the two other resonance techniques that take place here at the lab: nuclear magnetic resonance (NMR) and ion cyclotron resonance (ICR). The big difference is that EMR looks at electrons rather than nuclei (which is the case in NMR) or ions (in the case of ICR).
There are several forms of EMR, including electron paramagnetic resonance (EPR), electron spin resonance (ESR) and electron cyclotron resonance (ECR).
The big difference between ESR and NMR is that nature actually likes to pair up electrons (this also happens in nuclei, but not to the same extent). For the vast majority of materials, the electrons pair up so that one of them has its spin pointing one way, and the other has its spin pointing in the opposite direction. In other words, for every tiny magnet pointing one way, there is another pointing in the opposite direction. As a result, the substance has no net spin and no magnetism. In this situation, you cannot do an ESR or EPR experiment.
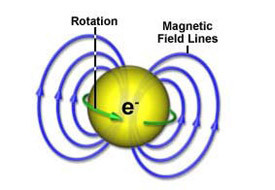
So what is the spin of an electron? Most fundamental particles in nature have a property called angular momentum. In the case of an electron, this property gives the impression to the outside world that the electron is actually spinning. Thus, when electrons interact with each other, or with electromagnetic radiation (photons), they can exchange angular momentum and energy. It is through such exchanges that we can learn what goes on inside atoms, molecules and solids. For EMR, we use microwaves to look at the electrons in solids.
Most importantly, an electron has a negative charge. When charged objects spin, they produce magnetism! In other words, a spinning electron behaves like a tiny magnet. Indeed, THIS IS the origin of magnetism, with the electron representing the fundamental magnetic particle. It is on this tiny "magnet" that we perform the ESR or EPR experiments.
However, in magnetic substances, not all of the electron spins are paired. For this reason, EPR is a very powerful tool for studying magnetism. This is of huge technological importance, given the significant role magnets play in our everyday lives, e.g. in electric motors and the memory in our computer hard drive. EPR/ESR is also useful to chemists who can deliberately attach unpaired electrons to molecules, a process called spin labeling. They can then do EPR on that labeled molecule, using that unpaired electron as the local probe that reveals what's going on inside the molecule.
EMR is one of the most interdisciplinary fields in the magnet lab, because we study everything from condensed matter physics to biology and chemistry.


