What is low temperature physics? Imagine what it's like to drive through a dense fog. There are structures out there – buildings, trees, pedestrians -- but you can see these shapes only dimly, if at all.

MagLab staffer Eun Sang Choi (left) assists West Virginia University scientist James Rall with an experiment in the Millikelvin Facility.
That's what it's like for physicists who want to observe certain quantum mechanical properties – behavior that occurs at the atomic level. At normal temperatures, they can't see these behaviors. But if they make their experiments very, very cold, it's as if a mist dissipates, revealing a clearer view of what's going on.
Invisible to the naked eye, atomic particles are constantly jostling, wiggling and zipping inside all materials, getting in the way of what the scientists want to see. When you cool the particles down, however, they slow down: Liquid water molecules have less energy than molecules of water vapor, and solid ice holds less energy than water. When a material is cold enough, scientists can see some really neat stuff: heavy fermions, the quantum hall effect and exotic phase transitions.
It takes a lot more than a Frigidaire to sedate atoms sufficiently for this kind of study. The fields of low-temperature and ultra low-temperature physics deal in degrees far below anything found in the natural universe – way down to almost -273 degrees Celsius (-460 degrees Fahrenheit). Scientists, though, prefer a different temperature scale called Kelvin. This scale literally starts from zero: 0 K (absolute zero) is as cold as cold can be, a condition that has not even been achieved in a laboratory.
We have, however, come pretty close. As a matter of fact, two facilities at the National High Magnetic Field Laboratory are dedicated to creating extremely low temperatures on a daily basis, so that scientists can see what happens when a material is cooled to the point of almost no thermal motion, then put inside a strong magnetic field. The particles in the material respond to the magnetic field in a way that can reveal fascinating secrets about matter.
At the MagLab's Millikelvin Facility in Tallahassee, scientists conduct sensitive experiments at temperatures within 7 thousandths of a Kelvin above absolute zero. If that number doesn't give you a brain freeze, try this: In the MagLab's High B/T Facility, located at the University of Florida's Microkelvin Laboratory in Gainesville, experiments can be conducted within 40 millionths of a Kelvin above absolute zero.
| Decimal |
Exponential |
Prefix |
| 0.1 (tenth) |
10-1 |
deci |
| 0.01 (hundredth) |
10-2 |
centi |
| 0.00.1 (thousandth) |
10-3 |
milli |
| 0.000001 (millionth) |
10-6 |
micro |
| 0.00000000.1 (billionth) |
10-9 |
nano |
| 0.000000000001 (trillionth) |
10-12 |
pico |
How do you create such a frigid environment?
The How: Zeroing in on Zero
Two tools make such low temperatures possible: dilution refrigerators and adiabatic nuclear demagnetization refrigerators. The former gets experiments colder than you can imagine. The later gets them even colder.

Dilution Refrigerator
Dilution fridges (dil fridge for short) owe their cooling power to the incredible element helium and its two isotopes, helium-3 (3He) and helium-4 (4He). Though most people are familiar with it as the gas inside their party balloons (4He), helium can also condense into a liquid – but only at 4.2 K. This property makes helium a very valuable cryogen in science. Using a condensation/evaporation cycle not unlike that of a kitchen refrigerator, a dil fridge takes 4.2 K liquid helium way down to 1.5 K.
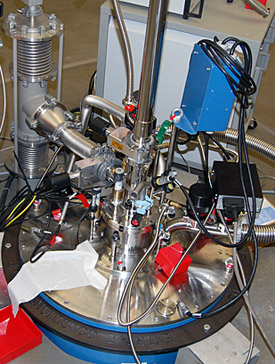
A dil fridge in the Millikelvin Facility.
That's no small feat. But low-temperature physicists are aiming even lower. By utilizing the special properties of helium isotopes and exploiting phase transitions, osmosis, evaporation, condensation and (of course) dilution, dil fridges gradually reach the millikelvin range. At the MagLab"s Tallahassee headquarters, dil fridges are used in the Millikelvin Facility's superconducting magnets and in the world-record 45-tesla hybrid magnet.
At the High B/T Facility, a dil fridge is only the first of a two-stage cooling process. The second stage involves an adiabatic nuclear demagnetization refrigerator – NDF for short.
An NDF doesn't use a refrigerant, such as liquid helium. Rather, it cools things down with a magnet and a bundle of metal, taking advantage of the fundamental properties of magnetism. The magnetization (M) of a material is uniquely dependent on the ratio of applied magnetic field (B) to temperature (T). If a magnet can be perfectly isolated, M (or B/T) stays constant. So when you lower B, you necessarily lower T – once, that is, you have created a strong initial magnetization (M). And that is the trick. For example, if you have a screwdriver that has become magnetized and you want to make it non-magnetic again, you can heat the metal and the ferromagnetism will go away. Conversely, if you want to make it a better magnet, you can decrease the temperature while exposing the screwdriver to an external magnetic field to increase the alignment of the magnetic domains within the metal.
What's High B/T?
The letter "B" refers to magnetic field and "T" stands for temperature. The High B/T Facility boasts the world record for the highest magnetic field-to-temperature ratio: 16.5 tesla / 0.004 Kelvin.
In an oversimplified nutshell, NDFs work like this. You start with a piece of copper. The nuclei of copper atoms have a property called spin: They randomly spin about an axis, roughly like the Earth around its axis. This spinning turns the nucleus of each atom, in effect, into a tiny magnet. So each copper nucleus has a north and south pole, just like a magnet. When you put the copper inside a high magnetic field, those nuclei will align with that field, their north and south poles lining up with the poles of the stronger external field.

Scientists at work at the High B/T Facility.
When you then reduce the applied magnetic field, the nuclei want to fall out of alignment and move randomly around again (i.e., increase entropy). However, M (or B/T) of a perfectly isolated sample must remain constant, so as the applied field is lowered (controlled from the outside by the scientist), the temperature of the copper and of the experiment (connected to the copper by a solid silver cylinder) is also lowered. This is nuclear demagnetization. (The reason for the long, silver extension is that the experimenter may want to apply a high field to the sample itself while holding the nuclear refrigerator cold. The geometry of the High B/T Facility, along with a special, central superconducting coil to shield the experimental region, allow the scientists to do just that.) The perfect isolation needed to carry out nuclear demagnetization is achieved with the use of a superconducting heat switch placed between the dilution refrigerator stage and the NDF.
In this way, the NDF takes the experimental space down from the millikelvin range of the dil fridge to the microkelvin range, cooling the space by a factor of 1,000.
After the NDF has done its job, the system will slowly warm up a little due to vibration, weak radio signals from the neighborhood and residual heat leaks via the very wires needed to make measurements at the sample. Luckily, these systems are carefully designed to absorb this heat while remaining cold enough for the experiment. For the High B/T facility, this cooling capacity is about one milliwatt. To appreciate how little heat that is, think of your typical 100-watt light bulb – then divide by a million. That's a nanowatt. As High B/T Director Neil Sullivan likes to explain it, that's about the amount of energy produced by a Florida mosquito doing one push-up per second.
This high capacity compared to other facilities enables researchers to stay at the extremely low temperatures for several days, even weeks in some cases.
Seems like an awful lot of trouble to go to for cooling some small objects. Not to mention cost: At High B/T, each experiment uses about 1,000 liters of helium just to get started, then about 250 liters per week while experiments are conducted. And helium ain't cheap. But for low-temperature physicists, it's well worth it. Why?
The Why: Quantum Effects and Freaky Fermions
Many of the physicists using the lab's Millikelvin Facility are studying the fractional quantum Hall effect. This is a still-mysterious variant of the conventional (integer) Hall effect, in which a voltage is produced across some materials when a magnetic field is applied. In the fractional quantum Hall effect, special resistance-free electrically conducting states occur for ultra-thin sheets of semiconductors when a specific magnetic field is applied. These states, observed in two-dimensional structures, correspond to new states of matter. Some fractional quantum Hall states may one day be used as qubits in future quantum computers.

University of Pittsburgh scientist Daniela Bogorin adjusts an experiment on the fractional quantum hall effect in the Millikelvin Facility.
Others physicists, like the MagLab's Tim Murphy, are interested in heavy fermions, such as CeCoIn5. In these intriguing materials, electrons appear to be much heavier than they actually are due to the interactions between the electrons and the crystal structure of the material, resulting in unusual behaviors.
"Usually, magnetism destroys superconductivity," explains Murphy, who directs the Millikelvin Facility. "But in some heavy fermions, magnetism not only coexists with superconductivity, it actually enhances the superconductivity. That flies in the face of our traditional understanding of superconductivity, and reminds us we don't understand as much as we think we do."
The High B/T Facility attracts scientists who study special phase transitions. These are not the run-of-the-mill transitions we're familiar with, such as water freezing to ice on a cold day, or hot tea vaporizing. Rather, these are exotic phase transitions that happen either at really low temperatures, or at the combination of very high fields and very low temperatures. These include Bose-Einstein condensates, superfluids (fluids that flow without any friction) and supersolids, a phase that has been theorized but not yet fully demonstrated.
Physicist Moses Chan of Pennsylvania State University, for example, has been studying pure helium-4 at High B/T, where he has observed behavior that suggests it is, at very low temperatures, a supersolid. Further experiments are underway, using innovative nuclear magnetic resonance techniques and specialized equipment, that may confirm whether this supersolid actually exists.
By no means are such experiments for the faint of heart.
"They're large, they're complicated and they can take a long time," says Sullivan, the High B/T director. "They are very costly experiments."
One experiment can last for months, as scientists painstakingly map out data points at different temperatures and magnetic fields. Because rapid changes in magnetic field create heat in the system, adjustments must be made very, very slowly.
"It's just the nature of the physics," says Murphy. "It has to be slow, there's just no other way. As soon as you go fast – oops – there's your temperature."
As little as an extra nanowatt of heat (remember that push-up pumping mosquito?) can undo a costly experiment many months in the planning. For that reason, all the electronics associated with an experiment – the wires and coils that measure what's going on -- are meticulously designed and built to generate next to no heat.
Working at the cutting edge of science and technology always entails risks.
"Many experiments fail," says Sullivan. "When you cool things down, there's lots of stress and strain, and sometimes things break or don't work well, or the sample itself is faulty." Many High B/T experiments are first tested under less demanding conditions to see if they are likely to hold up to the extremes of high fields and low temperatures.
Electronics are not the only nemesis of low-temperature experiments. Vibrations can also create heat that sensitive experiments can't tolerate. Both the Millikelvin and High B/T facilities were designed with this in mind. At High B/T, for example, a 35-foot concrete tripod supports the magnet system, each foot encased in 10 tons of concrete. Atop this tripod sits a shock mount, a kind of pneumatic isolation pad that further isolates the system from ground vibrations.
In addition, both low-temperature facilities are surrounded by shields that prevent external electromagnetic radiation, such as radio waves, from disrupting sensitive equipments.
So next time you're hot and bothered by summer weather, remember the ultra-cold microclimates being created inside magnets at two laboratories in Florida – one of the hottest states. Thinking of experiments that cool will make you feel positively nippy!
By Kristen Coyne