Helium, helium, everywhere,
Nor any drop to drink.
Physics Factoid
Helium – less soluble in human blood than nitrogen – is combined with oxygen to create a nitrogen-free atmosphere in decompression chambers for deep sea divers so that they don’t get the bends.
Our apologies to Samuel Taylor Coleridge, but this twist on the famous line from “The Rime of the Ancient Mariner” seemed a fitting start to a discussion of cryogenics – the art and science of keeping things frigid.
That’s because helium – the most important cryogen used in science – is both abundant and hard to come by (a riddle we’ll clarify in a bit). We don’t think Coleridge would begrudge us a little artistic license, especially since our story, like his mariner’s tale set in the Antarctic, takes place in extreme cold.
Cryogenics isn’t just about helium. By definition, it’s the study of very low temperatures (colder than any place on Earth), how to produce and exploit them, and how materials behave at those temperatures. But helium is of special interest. Thanks to its unique properties, it can be used to make other stuff really cold. It is the ultimate deep freezer: At the Magnet Lab it keeps our powerful superconducting magnets at the extremely low temperatures they need in order to function.
Looking at these incredible magnets from the outside, all you see is a metal cylinder, perhaps several scientists tall. You may not realize that most of what you see is not the magnet itself, but the insulating chambers filled with liquid helium and another important cryogen, liquid nitrogen. Take a look below to see one of these machines exposed.

The MagLab uses about 100,000 cubic feet of helium every week. That’s gas helium. To be of any use, though, it must be turned into elusive, precious “drops,” as in Coleridge’s poem. But before we get into that mystical transformation, let’s take a few minutes to consider liquid and the other states of matter … and revisit Coleridge’s gruesome ballad. The British bard will, in fact, accompany us throughout our story, a reminder that science and art need not always seem so far apart...
The State of the Matter
And now there came both mist and snow,
And it grew wondrous cold:
And ice, mast-high, came floating by,
As green as emerald.
Most of us remember from science class that there are three basic states (or phases) of matter: gas, liquid and solid. As our Ancient Mariner illustrates, water is the most familiar example of this: You can evaporate it into a gas, drink it as a liquid, or freeze it into a solid cube of ice. And to go from one state to another, you have to change the temperature.
We could get fancy here and talk about other exotic states of matter that scientists have created in laboratories, such as Bose-Einstein condensates and fermionic condensates. But for our purposes we’ll stick with gas, liquid and solid.
Physics Factoid
The fusion of hydrogen into helium provides the energy of the hydrogen bomb.
The important thing to remember is that when you move from one state of matter to another, you’re either adding or subtracting heat (i.e., energy). That means, in part, that atoms and molecules behave differently, depending on what state they’re in. Gas atoms, which exist at the highest temperatures, are the athletes of the group, solids are the couch potatoes at the low-end of the temperature scale for any particular material, and liquid atoms are in between. The athletes require more space to run around in, while the couch potatoes don’t mind sitting neatly in a row, crammed together on the sofa. It’s the whole heat expands, cold contracts thing.
Of course, not all atoms of a particular solid – let’s take for example a piece of aluminum – are vibrating at the same speed. If the solid is warm, they’re vibrating faster. If the solid is very cold, they’re slower. In many substances, when the atoms reach a specific critical temperature, they become quite sedate. This is what makes superconducting current possible: the electrons flow through the material with no resistance from the atoms (though the atoms continue to vibrate a little bit). That means there’s no heat generated in the process, as occurs in normal electricity. That’s the current that fuels the superconducting magnets.
The threshold between solid and liquid, of course, is the freezing point; between liquid and gas, it’s the boiling point. Different elements and compounds change states at different temperatures. Some elements don’t boil – i.e., go from liquid to gas – until well over 5,000 degrees Celsius ( 9,000 degrees Fahrenheit), hotter than some parts of the sun.
Helium is at the other extreme. It has the lowest known boiling point of any element, just a tad above the absolute coldest that anything can be.
Isn’t that special? We think so. Helium is special for a lot of other reasons as well. To talk about that, let’s make a dramatic temperature shift (again courtesy of Coleridge) from the nether regions of the thermometer to a much hotter spot.
Something Special about Helium
All in a hot and copper sky,
The bloody sun, at noon,
Right up above the mast did stand,
No bigger than the moon.
Helium is one of the main ingredients in our sun. It’s cooked to perfection inside the core of the sun (as well as other stars) in a recipe called nuclear fusion: Two hydrogen atoms coalescing into a helium atom.
In fact, “helium” comes from the Greek word for sun (helios) and was named in the late 1800s, when it was discovered. The element was first spotted by an astronomer studying a solar eclipse; he noticed a bright yellow emission line that didn’t correspond to any known element.

Turns out the “new” element was as old as the Big Bang. Further study determined that it was quite light; the second lightest element around, after hydrogen. Its atomic number is a measly 2 – meaning it is home to two protons, two neutrons and two electrons. We’re talking extremely low density. As you’ve witnessed in many a birthday balloon, it’s lighter than air. This is one of helium’s nifty features.
Another distinguishing feature of helium is that it belongs to a category of elements from the Periodic Table called inert gasses. These guys are like old bachelors, set in their ways, very much disinclined to hook up with other atoms. Helium is the inertest of the inert. While other elements feature electron distributions that predispose them to binding with other atoms to form molecules, life for the helium atom feels complete with its own two electrons contentedly orbiting. These fuddy-duddies like things the way they are, and they like their space.

Fellow featherweight hydrogen provides an interesting contrast. It feels incomplete with its single electron and seeks companionship either with other hydrogen atoms (H2) or by forming a trio with another hydrogen atom and an oxygen atom, an arrangement also known as water. Once it finds this bond, hydrogen is loath to let it go. That’s why, compared to other substances, water has such a high boiling point (100 degrees Celsius, or 212 degrees Fahrenheit): The hydrogen molecules in water are quite attached to each other and hold on as long as they can.
Physics Factoid
Ever hear of alpha particles? They’re helium nuclei!
Why else is helium special? It’s the only element capable of becoming, at super low temperatures, a superfluid, an amazing state akin to superconductivity in solids. While superconductive solids can carry an electrical current without resistance, a helium superfluid can flow unhampered by any viscosity whatsoever; it even flows up! This property, much studied at the MagLab and elsewhere, will be discussed later.
Light weight, inert, low density: Pretty neat element! But as far as making it a super cryogen, one more property is needed to clinch the deal.
Cold Enough for Ya?
The ice was here, the ice was there,
The ice was all around:
It cracked and growled, and roared and howled,
Like noises in a swound!
As we just learned, helium is the product of the sun’s nuclear fusion. But it’s a loooooong trip down the thermometer (way past Coleridge’s ice storm) before you get from the heat of the sun’s core – (millions of degrees, whether Fahrenheit or Celsius!) to the temperature at which helium will finally cease being a gas and turn into a liquid. That point is just a wee bit above absolute zero – the absolute coldest possible temperature. (Scientists, by the way, tend not to talk in terms of cold, but in terms of heat and the lack thereof; cold is simply the relative absence of energy in the form of heat. At absolute zero, there is absolutely no heat.)
Physics Factoid
Unlike helium, another cryogen, nitrogen, is abundant on Earth. In fact, it makes up about four-fifths of every breath you take!
Scientists, especially in cryogenics, talk about temperatures in terms of the Kelvin scale (plain old “K” for short), which starts at absolute zero (0 K). Helium boils at 4.2 K (-452.11 degrees Fahrenheit, or -268.95 degrees Celsius). That can be a little hard to picture, when everything else on the planet, at that temperature, has long since frozen solid. And unless you really subject it to high pressure – another variable that can affect phase changes – helium will never, ever even think about turning into a solid.
Not that you would find 4.2 K on Earth, except in a laboratory. In fact, those temperatures don’t even exist in our solar system. Far-flung Pluto, 38 K at its very coldest, is balmy in comparison. The table below gives an idea of the range of temperatures in the universe, depicted in the three common scales used to measure them.
| Location |
°C |
°F |
K |
| Sun's Core |
15.5 million |
27 million |
15.5 million |
| Temperature required for thermonuclear fusion of hydrogen |
2.8 million |
5 million |
2.8 million |
| Earth's Core |
7,277 |
13,040 |
7,500 |
| Surface of Venus (the hottest planet), at its hottest |
467 |
872 |
740 |
| Sun's Surface |
5,538 |
10,000 |
5,811 |
| Moon's surface, at its hottest |
127 |
260 |
400 |
| Boiling point of water |
100 |
212 |
373 |
| Highest temperature ever recorded on Earth (Libya, 9/31/1922) |
58 |
136 |
331 |
| Highest temperature ever recorded in US (Death Valley, 7/10/1913) |
56.7 |
134 |
330 |
| Average year-round temperature in Tallahassee, FL |
20 |
68 |
293 |
| Freezing point of water |
0 |
32 |
273 |
| Lowest temperature ever recorded in US (Alaska, 1/23/71) |
-62 |
-80 |
211 |
| Lowest temperature ever recorded on Earth (7/22/83, Antarctica) |
-89.2 |
-128.6 |
184 |
| Moon, at its coldest |
-173 |
-280 |
100 |
| Liquid nitrogen |
-196 |
-321 |
77 |
| Pluto, at its coldest |
-235 |
-391 |
38 |
| Liquid helium |
-269 |
-452 |
4.2 |
| Space between galaxies |
-270 |
-454 |
3.15 |
| Absolute Zero |
-273 |
-460 |
0 |
Now that you appreciate the temperature extremes we’re talking about, you can begin to understand the effort it takes to make liquid helium.
First off, though abundant in our sun and other stars (in fact, after hydrogen, it is the second most abundant element in the universe), helium is relatively rare on Earth. Even after you do find the gas, you’ve got to turn it into liquid, into Coleridge’s precious drops, to get it cold enough for our magnets.
A Recipe for Liquid Helium
Let’s leave the MagLab for a little field trip to find out more about where our helium comes from.
Welcome to the amber waves of grain of Kansas. It is here and in several other Great Plains states that our quest for liquid helium begins. It’s one of a few spots in the world where helium gas can be found in relative abundance, if you dig in the right place.
Natural gas contains helium. Though the concentrations are small – usually less than 1 percent – it’s nonetheless much more plentiful there than in our atmosphere. Helium can be distilled out of natural gas, then shipped to the MagLab (not to mention countless party supply stores) in high-pressure tube trailers.
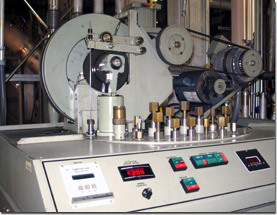
This helium liquefier services the world's strongest magnet – the MagLab's 45 tesla hybrid.
What do you need in order to turn that gas into a liquid? Why, a helium liquefier, of course. The Magnet Lab has three. Two of them are part of a closed system that recycles helium used by the world’s most powerful continuous field magnet, a 45 tesla hybrid that is part superconductive, part resistive electromagnet. (Tesla is a measure of magnetic field: 45 tesla is one million times the Earth’s magnetic field.) The magnet draws scientists from across the globe who use it for experiments, and a considerable amount of helium is needed to keep the superconducting part going around the clock. The magnet is not in use all the time, but is always kept at cryogenic temperatures (except for maintenance and repairs) because it takes more than a month to get from room temperature down to operating temperature.
The MagLab’s third helium liquefier services the rest of the facility, including a 21 tesla, 900 MHz superconducting magnet used by scientists for nuclear magnetic resonance.
The transformation from a room-temperature gas to a very cold liquid is less like a slippery slope downward and more like a hard climb up. It takes a fair amount of human effort to get those atoms to scale back on all their effort. Remember all those athletic gas atoms we discussed earlier? Well, the trick is to get them to slow down and relax. Because as they chill out, they – well, they chill out! Less work = less heat.
Our Magnet Lab specialists do this using a cyclical process of compression and expansion. First comes a one-two punch called isothermal compression: We cool the helium down with liquid nitrogen while at the same time compressing it.
Physics Factoid
Helium was first liquefied in 1908 by Dutch physicist Heike Kamerlingh Onnes
The liquid nitrogen does its job through heat transfer – the same idea that’s behind cooling down a warm soda by sticking it in the fridge. At 77 K (-320 degrees Fahrenheit), the liquid nitrogen absorbs the helium’s heat, carrying it off in evaporation.
The gas is then allowed to expand into a larger area. Those helium atoms do work as they spread out; in so doing, they give off heat and cool down. This cycle is repeated several times as the helium gets colder than an Alaskan winter, frostier than Antarctica at its worst. Down, down, down goes the temperature, from about 300 K (room temperature) to, eventually, about 4 K, when the helium finally yields and turns from gas to precious drops.
In some liquefying systems (such as the Magnet Lab’s), helium is used to cool helium. In other words, the incoming helium – the stuff you’re trying to liquefy – is exposed to the outgoing helium, which has just finished its shift cooling the superconducting magnet. Though the outgoing helium is “warmed up” by now and gaseous, it is still chilly enough to draw heat from the incoming helium.
Very cool, huh? (Now that’s an understatement!)
So, why is it we need all this helium again?
Some Like it Cold
The lab’s cyrogenics department maintains a fleet of 44 portable dewars – specially insulated containers that keep the liquid helium c-c-c-cold. These dewars are ubiquitous at the MagLab: scientists and students gingerly wheel them around to their experiments like proud mommas pushing perambulators.
The helium is used for three main reasons.
We’ve already explained the most important use: running the magnets, particularly our 45 tesla hybrid magnet and the 900 MHz NMR magnet. The superconducting part of these instruments must have an ambient temperature of less than 2 K to keep running.

Helium billows from a quenched superconducting magnet.
What happens, you may be wondering, if the temperature creeps higher? The dreaded quench. This is not the kind of quench that involves Gatorade or iced tea. It’s the consequence of the temperature rising above the minimum required to create superconductivity in the coils carrying the current that powers the magnet. If even a single coil among the 4 miles worth of coils in the magnet loses superconductivity because it gets too warm, the magnetic field will begin to break down in a series of unfortunate, domino-like events that works like this: When that segment stops superconducting, it begins conducting electricity normally instead – which means it gives off heat in the process. That heat will in turn increase the temperature of the adjacent coils, which in turn stop superconducting and begin to conduct electricity normally, generating more and more heat and eventually forcing the magnet to shut down completely, possibly damaging it.
The second use for helium at the lab is to cool down some of the experiments that go into the magnets. These ain’t no horseshoe magnets. They’re shaped more like a dowel with a small hole drilled through the center. That center is called the bore and that’s where the experiment goes, because smack dab in the middle of things is where the magnetic field is most intense.
Physics Factoid
Though we’ve focused on liquid helium and mentioned liquid nitrogen in this Magnet Academy class, there are two other important cryogens: liquid hydrogen and liquid oxygen, the latter of which is a very pretty shade of pale blue and is attracted to magnets!
The third and last use of helium here is as a fascinating subject of study in its own right. Researchers at the Magnet Lab conduct many experiments on this element at facilities that include the Liquid Helium Flow Facility, the Cryogenic Helium Experimental Facility and the Cryogenic Flow Visualization Apparatus. It is being studied to find ways to make better use of liquid helium as a superconductor cooler, and as a fascinating state of matter that scientists still only partially understand. Steven Van Sciver, a mechanical engineering professor and Magnet Lab scientist, has been investigating how superfluid helium can be used to separate extremely tiny particles. His work is of great interest to the pharmaceutical industry, as it could lead to improving drugs that are inhaled.

Technical Designer Bob Carrier readies a 500 liter dewar full of liquid helium for lab use.
One fascinating aspect of helium that intrigues scientists is superfluidity. At 2.17 K, helium completely loses its viscosity. Left in a closed ring, it could flow without stopping: There is no friction, much as a superconducting current encounters no resistance. It gets even curiouser. This superfluid conducts heat perfectly. As a result, it actually rises up the sides of an open container and flows over the top. You can even make a fountain out of the stuff simply by heating it even slightly.
That kind of gravity-defying magic is almost as eerie as the sailors in Coleridge’s ballad who, though lifeless, manage to get up and usher their boat out of the icebergs and back to (relatively) tropical England. In fact, why not close our story with that very scene, as the poet evokes the same sense of mystery and awe often inspired by science.
They groaned, they stirred, they all uprose,
Nor spake, nor moved their eyes;
It had been strange, even in a dream,
To have seen those dead men rise.
Thanks to the Magnet Academy's scientific advisers on this article. They were Steven Van Sciver, a professor of mechanical engineering with the Florida A&M University/Florida State University College of Engineering and an expert in cryogenics at the MagLab, and John Pucci, former Coordinator of Research Programs and Services for the MagLab's Cryogenic Operations.
By Kristen Coyne