Imagine a set of Tinkertoys — wooden nodes and colorful struts — so small that each piece is an individual molecule. Think of the endless variety of things you could build with it. Now imagine a kitchen sponge so porous that if you could somehow smooth out all the nooks and crannies it would cover 70 soccer fields. Think of how much it could sop up.
Although it may sound like a dream from over the rainbow, these two ideas actually describe the same thing: metal-organic frameworks (MOFs), a new kind of material with exciting applications from clean energy to data storage. But to make use of these materials, scientists first have to understand how they work, and that’s where high magnetic fields are helping out.
First studied in the 1990s, MOFs have seen an explosion of interest in the last two decades. The Tinkertoy-like nodes are made of one or more metal atoms (the "metal" in MOF), while the long struts are made of carbon-containing molecules (the "organic"). The struts can be quite long for molecules, resulting in materials riddled with microscopic holes, channels and chambers. This makes them useful for absorbing gases for purposes from hydrogen fuel storage to carbon capture.
But it's not just their porosity that makes MOFs useful, says Monique van der Veen, an associate professor of chemical engineering at Delft University of Technology in the Netherlands. It's also their mind-boggling variety. Like many MOF researchers, van der Veen has also studied an older class of porous materials called zeolites. But because those are all based on the same combinations of aluminum and silicon, their uses are limited.
"However, with MOFs you have everything at your disposal," says van der Veen: Dozens of metallic elements can act as the metal nodes — copper, aluminum and zinc, oh my! — and an endless variety of organic molecules can serve as the linkers. This customizability, she says, makes the frameworks a playground for scientists like her.
MOFs can be customized to absorb a specific gas or tuned to respond to changes in temperature, pressure or their chemical surroundings. For example, a MOF could be designed to absorb carbon dioxide (CO2) from power plant emissions or even from the ambient atmosphere. Another could be designed to absorb and slowly release 1-MCP (a gas that prevents ripening) to keep produce fresh without refrigeration.
It's a wild, wonderful world of MOFs (apologies to L. Frank Baum) — so wild, in fact, that we'll need a map to understanding it better. So we invite you to slip on your ruby slippers and follow along the yellow brick road as we explore this magical, microscopic Land of MOFs. Because, readers: We’re not in Kansas anymore.
Along the way, we will encounter some fantastical figures: The Scientists of the East, North and West (using the National High Magnetic Field Laboratory, headquartered in Tallahassee, Fla., as our point of reference, where much of their science takes place). From their labs in the Netherlands, Ontario and California, these researchers (all working for good, not evil, of course) will be our guides, each using different ways to navigate inside MOFs.
Scientist of the North:
A high-field view of complex structures
Witches may fear the slightest drop of water, but many MOFs are born in it: The frameworks are usually synthesized in a liquid environment, which makes building MOFs quite a bit harder than linking together atoms and molecules like so many Tinkertoys. For MOFs to be any use, though, that solvent has to be removed.</p

Yining Huang
Image credit: Vinicius Martins
"When you take the solvent molecules out, that can cause a dramatic change, like a change in MOF structure or even a total collapse of the framework," says Yining Huang, a professor of chemistry at Western University in Ontario and our Scientist of the North. "In some cases the changes are very subtle, but the subtle changes can have an impact on the MOFs' performance."
Huang studies how gases or liquids absorbed into a porous solid interact with it, so MOFs and their extremely high surface areas fascinate him.
"The record is more than 7,000 square meters per gram," he says. "So in one gram of the stuff, you have more surface area than a soccer field."
One of the most important tools Huang uses to understand these structures is nuclear magnetic resonance (NMR) spectroscopy. NMR works by jostling around a particular element in a material — say, carbon — with a strong magnetic field. As the atoms wobble under the magnetic influence, they emit signals that tell scientists what other atoms — Zinc? Hydrogen? Other carbons? — are bound to that atom and how they’re arranged. It’s the next best thing to Glinda’s crystal ball.
Researchers set up their NMR instruments slightly differently for each element, so someone like Huang can study each element in a MOF in turn.
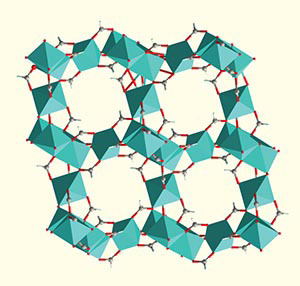
Huang studies the MOF alpha magnesium formate, shown here in its "activated" state, ready for adsorption with empty pores.
Image credit: Jun Xu
"MOFs provide a dream for NMR people," says Huang. "You can look at the metal, you can look at the linker and you can look at what has been absorbed inside."
Each NMR experiment gives a panoramic view inside the MOF from a different atom's perspective, like a nanoscopic version of Google's Street View. But capturing those different views is challenging.
In 2017, Huang was grappling with one such challenge. He was using oxygen NMR to study a MOF by the ungainly name alpha magnesium formate, noted for its ability to selectively absorb CO2. Once removed from a liquid bath, this material has oxygen atoms sitting at no fewer than 12 distinct positions. But oxygen is notoriously challenging to study with NMR, and instead of 12 sharp signals, Huang saw two blurry ones. That was enough to tell him the general structure of the framework but not enough to see subtle changes, like those that might occur when the solvent is removed.
That's when Huang met Zhehong Gan, a research faculty member at the National MagLab. Gan offered Huang the chance to try his experiments on a new NMR machine at the MagLab that produces a field of 36 teslas — the strongest magnet used for NMR in the world. Since the resolution of an oxygen NMR experiment increases with the square of the magnetic field, this would tremendously improve the resolution in Huang's previous studies, allowing him to get all the signals from oxygen atoms in their different positions in the MOF.
"The high field provides us with so many opportunities," says Huang. "Besides the oxygen in this work, we're also studying some of the metals in our other frameworks, like zinc and zirconium. These are tough nuclei to study, so we're fortunate to have this opportunity to solve these tough structural problems."
Scientist of the East:
Tracking electron traffic

Monique van der Veen
If high-field NMR gives you the finest details of a MOF's structure, why would you ever need anything else? Well, imagine trying to find your way around a new place using street-level views alone. You can have as detailed a view of the yellow brick road as you like, but without an old-school map you’d have no hint of what strange features (A frozen tin man? A spooky forest?) pepper the landscape around it — and how those may affect your journey. Where you’d turn to a map to see how different places are stitched together, materials scientists turn to X-ray diffraction — the same tool that Rosalind Franklin used to take the first “pictures” of DNA’s structure.
Franklin's diffraction differs only in details from the X-ray tools scientists like Huang or van der Veen use on MOFs. A beam of X-rays is scattered off a crystal, producing a pattern of lights and shadow that can be used to understand its size and shape. Are the atoms stacked directly on top of each other or interspersed like bricks? How far apart do the metal nodes tend to be? But even with the most precise street map, you can still get caught in a traffic jam. So scientists need yet another tool, something akin to Google Maps' traffic layer. But instead of the flow of traffic, this tool reveals the flow of electrons.
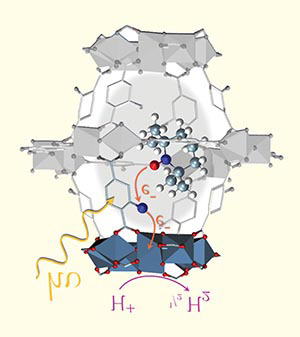
Monique van der Veen uses the interaction of light with a MOF to start chemical reactions of molecules inside the MOFs' pores. Here, a MOF called NH2-MIL-125 has adsorbed a molecule, the structure seen inside the gray MOF. Light (H+) is absorbed by the MOF, which induces the transfer of an electron from the molecule to the MOF.
Image credit: Jara Garcia-Santaclara
For van der Veen, our Scientist of the East, how electrons move through MOFs matters as much as their structure. She uses optical spectroscopy to provide this traffic layer-like insight, measuring how her materials react to light in the visible spectrum.
If light makes electrons jump from the MOF to the absorbed gas inside, that could be useful in more efficient chemical manufacturing: Since molecules are held together by shared electrons, adding even one electron to the mix can be all the impetus a molecule needs to make a new chemical bond.
Van der Veen also uses optical microscopy to measure piezoelectricity in MOFs: If bending a framework makes electrons create a voltage, then it’s piezoelectric and might be useful in generating green energy.
"I think in the next decade we will see MOFs moving into applications," says van der Veen. She predicts the first to be commercialized will likely simply absorb gas, but adds that, "I'm really interested to see how far we can get with conductive MOFs. They would open up a whole myriad set of new applications."
Scientist of the West:
Seeing through glassy MOFs
Mapping Manhattan's organized grid is a lot easier than making sense of London's tangle of streets. Similarly, many of these scientific mapping techniques only work on crystals: orderly, repeated lattices of atoms. Fortunately, most MOFs have a crystal structure.
But scientists like Sabyasachi Sen thrive in the disordered world of glass, which, like a swarm of flying monkeys, is disordered even at the smallest level, with each atom in a somewhat unique configuration. Because of this, glass is called an amorphous material, as are so-called glassy MOFs.

Sabyasachi Sen
Image credit: Courtesy of UC Davis
Sen, a professor of materials science and engineering at the University of California, Davis, and our Scientist of the West, has long worked with these wacky substances. He was originally trained in geochemistry, where he learned about the glass-like volcanic rocks that form when lava cools suddenly, and worked for many years at Corning, the glass company that invented Pyrex cookware and the Gorilla Glass that probably makes up your smart phone’s screen.
Amorphous MOFs have the same building blocks as their crystalline counterparts but arranged in a disordered network. So the porous structure of the MOFS can be preserved even when they are amorphous and could be used to store CO2 for carbon sequestration, says Sen, or hydrogen gas for fuel cells. Also, because these compounds contain metals, they make for a glass that's far more stable than one made from carbon-based molecules alone.
Despite his many years working with glass, it was only recently, with the aid of MagLab researchers, that Sen was able to prove that the zinc-based MOFs he works with were truly amorphous — even at the smallest level involving the disposition of neighboring nitrogen atoms around zinc.
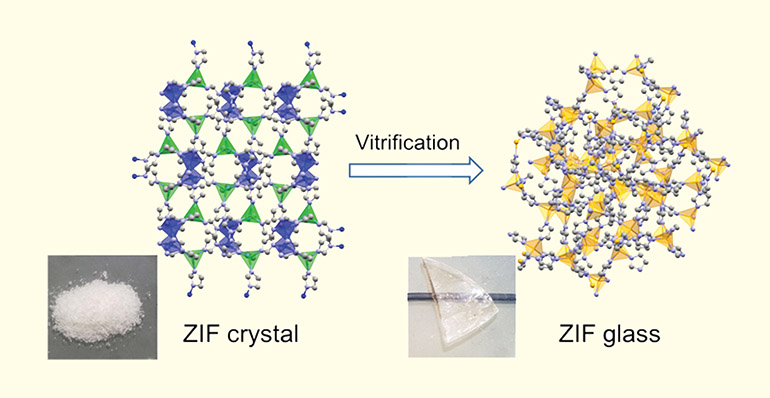
Sen studies amorphous MOFs, such as this zeolitic imidazolate framework. When turned into a glass, the structure retains the same building blocks, but now arranged in a disordered network. Yet the MOF remains porous, a property that could be exploited, among other things, for carbon sequestration in the future.
Image credit: R.S.K. Madsen, A. Qiao and Y. Yue from Aalborg University, Denmark; courtesy of Science.
"Zinc is very challenging to look at through NMR," says the MagLab's Gan. But the world-record magnet at MagLab helped Sen and Gan pull back the curtain and see the true, glassy nature of these MOFs. The MagLab's research team also applied neat NMR tricks, such as spinning the MOF like an airborne home in a tornado at a so-called "magic angle" or using custom radio wave pulses to perturb it just so to coax information out. "We basically throw everything in," Gan says.
Now Sen knows he's truly working with glassy MOFs and is looking forward to more high-field collaboration with Gan.
"Even though we've never met in person, we have more than 20 papers together," says Sen. "They're brilliant spectroscopists who are coming up with new NMR techniques all the time, and they're always willing to mentor my students in terms of processing and interpreting the NMR data."
The Wizard of MOFs:
Mobile molecules for data storage

Rob Schurko
Our final stop across this Land of MOFs will be the Emerald City itself: The MagLab's Tallahassee headquarters (a capital city known, in fact, for its stately live oaks, pines and palms). Given this majestic address, we dub our next scientist, director of the MagLab's NMR Facility, the Wizard of MOFs. As befits the wizard of the Emerald City, chemist Rob Schurko's MOFs are crystalline. But in some ways they're weirder even than glassy MOFs.
With the aid of some very careful chemistry, Schurko and his colleagues in the research group of Stephen Loeb at the University of Windsor make MOFs in which the long linkers have other molecules wrapped around them, like a witch astride a broomstick. So the rings are mechanically, rather than chemically, bound to the MOFs' structure — lending them the name mechanically interlocking MOFs. Depending on what else has been absorbed into the MOF, these ring molecules can rotate freely, wobble about and even shuttle from one end of the stick to the other. But as long as the MOF framework holds together, they won't leave it.
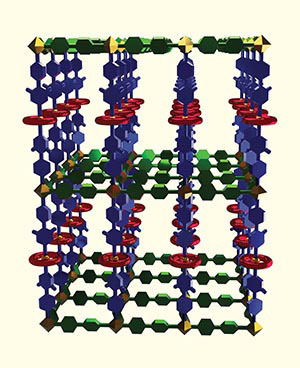
"Scientists are using MOFs like this one (called UWDM-3), featuring mechanically interlocked molecules, to design rudimentary switches, shuttles and machines at the nanoscale or molecular levels that could one day be used in applications like sensors, chemical separation, data storage or drug delivery.
Image credit: Benjamin Wilson, Stephen J. Loeb and Robert W. Schurko
This behavior could function as a Munchkin-size switch, says Schurko. The ring might move to one end of the linker in response to a specific stimulus — say a specific color of light — and to the other end in response to another.
"I think one of the grand goals would be something like data storage, where you have a 0 or 1 position literally down at the level of molecules," Schurko says.
There are other applications too. The rings provide a way to manipulate the shape of the pores so that the pores will preferentially absorb one gas over another. And if the rings can be convinced to move in reaction to the gas, and if that movement can be detected, then that MOF is suddenly a highly specific, low-energy gas sensor.
Schurko uses NMR to determine whether NMR spectra of the rings' atoms are sharp and clear — meaning the rings are held firmly in place — or "blobby," meaning the rings are free to wobble or rotate around the rod.
These experiments are much easier and more efficient when performed in high-field magnets, according to Schurko. "We have to do fewer experiments, and less fancy experiments, to get the signal we need," he says. "So, each experiment is more and more routine and reveals motions at the molecular scale that tell us a lot about how these materials work."
Close your eyes tight, click the heels of those ruby slippers together and repeat after me: When it comes to studying MOFs, there's no place like high fields.
More about MOFs
Want to learn more about MOFs? Check out these links to some of the research by the scientists featured in this story.
Yining Huang
- Vinicius Martins, et al, Higher Magnetic Fields, Finer MOF Structural Information: 17O Solid-State NMR at 35.2 T, J. Am. Chem. Soc. (2020).
- Shoushun Chen, et al, Anhydride Post-Synthetic Modification in a Hierarchical Metal–Organic Framework, J. Am. Chem. Soc. (2020).
- Shoushun Chen, et al, Cleaving Carboxyls: Understanding Thermally Triggered Hierarchical Pores in the Metal–Organic Framework MIL-121, J. Am. Chem. Soc. (2019).
- Shoushun Chen, et al, Probing Calcium‐Based Metal‐Organic Frameworks via Natural Abundance 43Ca Solid‐State NMR Spectroscopy, Chemistry A European Journal (2018).
Rob Schurko
- B.H. Wilson, et al, Precise Spatial Arrangement and Interaction between Two Different Mobile Components in a Metal-Organic Framework, Chem7 (2021).
- K. Zhu, et al, A Molecular Shuttle that Operates Inside a Metal-Organic Framework, Nature Chem., 7 (2015).
- V.N. Vukotic, et al, Mechanically Interlocked Linkers inside Metal-Organic Frameworks: Effect of Ring Size on Rotational Dynamics, J. Am. Chem. Soc., 137 (2015).
- P. Martinez-Bulit, et al, Solvent and Steric Influences on Rotational Dynamics in Porphyrinic Metal-Organic Frameworks with Mechanically Interlocked Pillars, Cryst. Growth & Design, 19 (2019).
- B.H. Wilson, et al, Exploring the Dynamics of Zr-Based Metal-organic Frameworks Containing Mechanically Interlocked Molecular Shuttles, Faraday Discussions (2020).
Sabyasachi Sen
- Rasmus S. K. Madsen, et al, Ultrahigh-field 67Zn NMR reveals short-range disorder in zeolitic imidazolate framework glasses, Science, 27 (2020).
- Thomas D. Bennett, et al, Melt-Quenched Glasses of Metal–Organic Frameworks, J. Am. Chem. Soc., 138 (2016).
- Ang Qiao, et al, A metal-organic framework with ultrahigh glass-forming ability, Science Advances (2018).
Monique van der Veen
- Jara G. Santaclara, et al, Organic Linker Defines the Excited‐State Decay of Photocatalytic MIL‐125(Ti)‐Type Materials, ChemSusChem (2016).
- Kamal Asadi, Monique Ann van der Veen, Ferroelectricity in Metal–Organic Frameworks: Characterization and Mechanisms, European Journal of Inorganic Chemistry, 27 (2016).
- J. G. Santaclara, et al, Understanding metal–organic frameworks for photocatalytic solar fuel production, CrystEngComm, 19 (2017).
Story by Bennett McIntosh