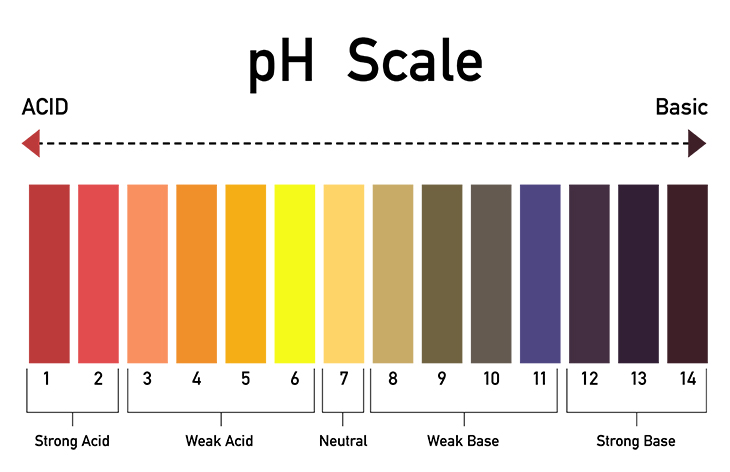
From our bodies to our Earth’s oceans and rocks, acids and bases play an important role in our lives and the environment around us. If you have tasted lemon juice or washed your hands with soap, you’ve experienced acids and bases. Scientists classify substances as acids, bases, or neutral, using what’s called the pH scale, which stands for “potential of hydrogen.” It measures the concentration of hydrogen ions, or charged hydrogen particles, in a substance. Acids have a pH level below 7 while bases have a pH level above 7.
Acids and bases are at the heart of many chemical reactions and are essential in various industries, including agriculture, medicine, and manufacturing.
A chemical reaction is like a recipe where different substances mix together and, instead of just combining, they change into new, different things. Just as when you bake a cake, the flour, sugar, and eggs combine to create a new substance with different properties.
Other examples include burning wood, where the wood combines with oxygen to create ash, smoke, and heat; rusting iron, where iron combines with oxygen and water to form rust; and photosynthesis, where plants convert carbon dioxide and water into sugars and oxygen.
MagLab scientists use high magnetic fields to study chemical reactions and chemical bonding with applications in many areas, including development of more efficient fuel, how to clean up toxins in our environment, and the role of various proteins in human health and disease.
In this exciting experiment, you can mix an acid and a base inside a balloon and see what happens.
What You’ll Need:
- A balloon
- Plastic food-service gloves
- Baking soda
- Vinegar
What You’ll Do:
1. Working with a helper, pour a spoonful of baking soda into the uninflated balloon.
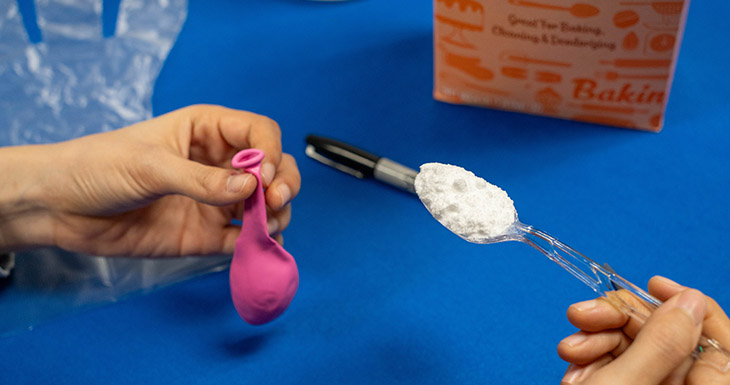
2. Cut a finger off the glove.

3. Pour 1-2 tablespoons of vinegar into the finger and tie it off.

4. Place a bag of vinegar inside the balloon. You might need a helping hand.

5. Tie off the balloon.

6. Use a marker to decorate your balloon.

7. Place the balloon on the floor. Gently step on it. Watch what happens!

Stepping breaks open the vinegar, mixing it with the baking soda. This causes a chemical reaction between oxygen and hydrogen in the two substances. The reaction releases carbon dioxide, which inflates the balloon. It also produces water, and a form of salt called sodium acetate.
Did you know?
- You are powered by chemistry. The food you eat is digested by a series of chemical reactions, breaking it down into small compounds that your body can use for energy.
- Batteries work because of chemistry. A chemical reaction between the electrodes and electrolytes produces a flow of electrons and converts chemical energy into electrical energy.
- Common table salt is made of two substances that are dangerous in their elemental forms. Sodium is highly reactive and can explode when mixed with water. Chlorine is a toxic gas. They combine in a chemical reaction to form salt, sodium chloride, becoming a stable compound and a flavor enhancer for many foods!