What did scientists discover?
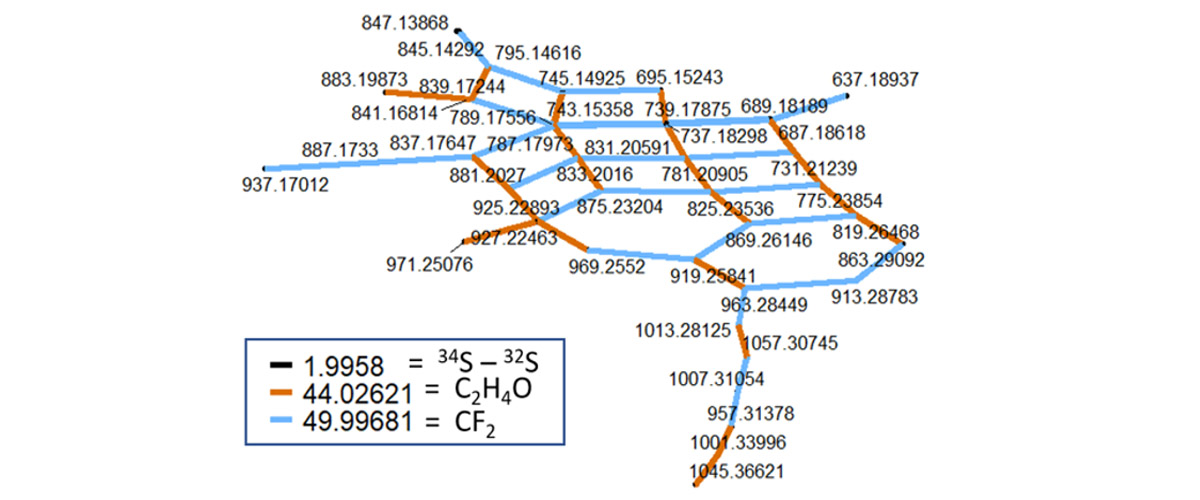
PFAS are mixtures of thousands of individual chemicals, some of which with significant toxicity. MagLab users and scientists teamed to develop a new method that can identify individual fluorinated environmental contaminants in mixtures consisting of thousands of other chemical compounds that are typically detected in natural water or soil samples.
Why is this important?
Thousands of man-made perfluoroalkyl and polyfluoroalkyl substances (PFAS) contaminate our planet from the North to the South Pole. PFAS are known as "forever chemicals" because they do not break down in the environment. Only about 15-50 individual PFAS species are typically measured during water, soil, and food analysis. However, those PFAS that are yet unknown ("dark matter") may be more harmful or spread faster in the environment than the few that are analyzed on an every day basis. This newly developed method will allow other scientists to take a closer look at how more of these PFAS chemicals move into water, soil, plants, feedstock, and eventually into humans, and ultimately at the risks they pose.
Who did the research?
Robert B. Young1, Nasim E. Pica2, Hamidreza Sharifan2, Huan Chen, Holly K. Roth2, Greg T. Blakney3, Thomas Borch2, Christopher P. Higgins4, John J. Kornuc5, Amy M. McKenna2,3, Jens Blotevogel2
1New Mexico State University; 2Colorado State University; 3MagLab; 4Colorado School of Mines; 5NAVFAC EXWC
Why did they need the MagLab?
PFAS identification relies on measuring differences in weight between individual molecules. Only the Fourier-Transform Ion Cyclotron Resonance Mass Spectrometers at the MagLab can distinguish among the many PFAS that differ in mass by only a 10-trillion-quadrillionth fraction of an ounce.
Details for scientists
- View or download the expert-level Science Highlight, A Deep Dive Into Forever Chemical Dark Matter
- Read the full-length publication PFAS Analysis with Ultrahigh Resolution 21T FT-ICR MS: Suspect and Nontargeted Screening with Unrivaled Mass Resolving Power and Accuracy, in Environmental Science and Technology
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779); Jens Blotevogel (SERDP ER20-1265)
For more information, contact Christopher Hendrickson.