What did scientists discover?
Organic acids in petroleum are known to corrode iron pipes in oil refineries. However, the corrosion rate does not correlate well with the acid content of the oil, which suggests that only a fraction of the acids contribute to steel corrosion. This new method to characterize the initial acids and the subsequent products formed from acid corrosion can reveal the reactive acid species. This information can be used to better improve oil valuation and refining.
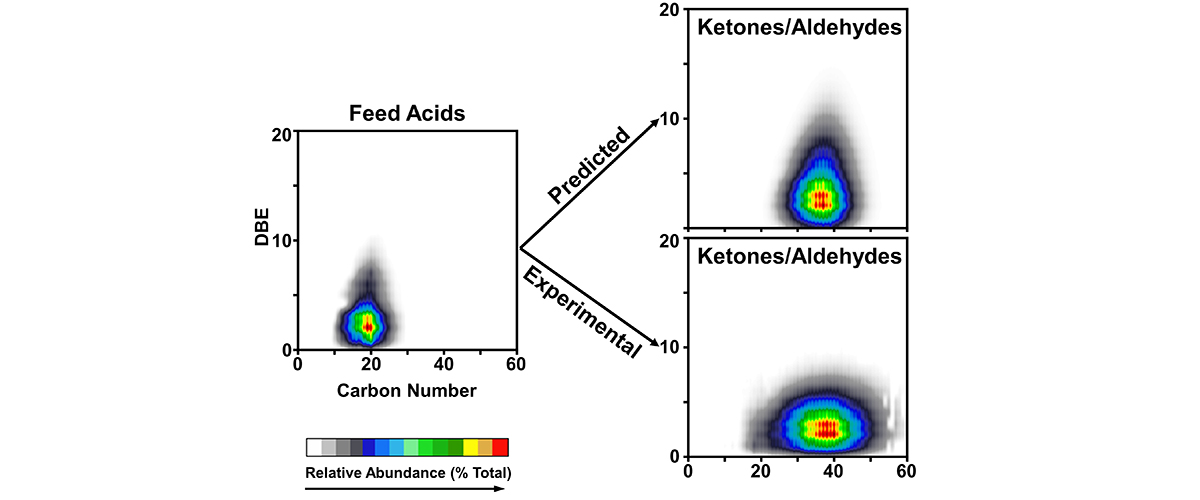
We show that corrosion in acidic crude oils depends on the specific structures of the acid molecules. Acids with fewer rings and double bonds in their structure are more corrosive.
Why is this important?
"Opportunity crudes" are those crude oils available at discounted price due to higher concentration of organic acids (naphthenic acids). Naphthenic acids cause corrosion in the steel pipes of oil refineries at a cost of ~ $1 billion annually. The acids react with iron in the steel to form oil-soluble iron naphthenates, which decompose at refinery temperature to yield oil-soluble ketones and oil-insoluble iron and magnetite (Fe3O4). Each ketone is composed of the two acids that initially reacted with iron to form the iron naphthenate, so analysis of the ketones formed reveals which specific naphthenic acids reacted with iron. Not all acids are equally corrosive, so this knowledge can be used to provide more accurate oil pricing and better refinery strategy.
Who did the research?
Krajewski, L.C.1,2, Robbins, W.K.2, Corilo, Y.E.1, Bota. G.4, Marshall, A.G.1,3, and Rodgers, R.P.1,2,3
1ICR User Facility, National High Magnetic Field Laboratory 2Future Fuels Institute (FFI) 3Department of Chemistry and Biochemistry, Florida State University 4Inst. for Corrosion and Multiphase Tech, Ohio University
Why did they need the MagLab?
There are tens of thousands of different naphthenic acids, so the ultrahigh resolution mass spectrometers at the Maglab are required to resolve and identify the original unreacted naphthenic acids and the ketone products.
Details for scientists
- View or download the expert-level Science Highlight, Understanding Oil Refinery Corrosion Caused by Acidic Crude Oils
- Read the full-length publication, Characterization of Ketones Formed in the Open System Corrosion Test of Naphthenic Acids by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry, in Energy Fuels
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1157490), R.P. Rodgers (FFI)
For more information, contact Chris Hendrickson.