What did scientists discover?
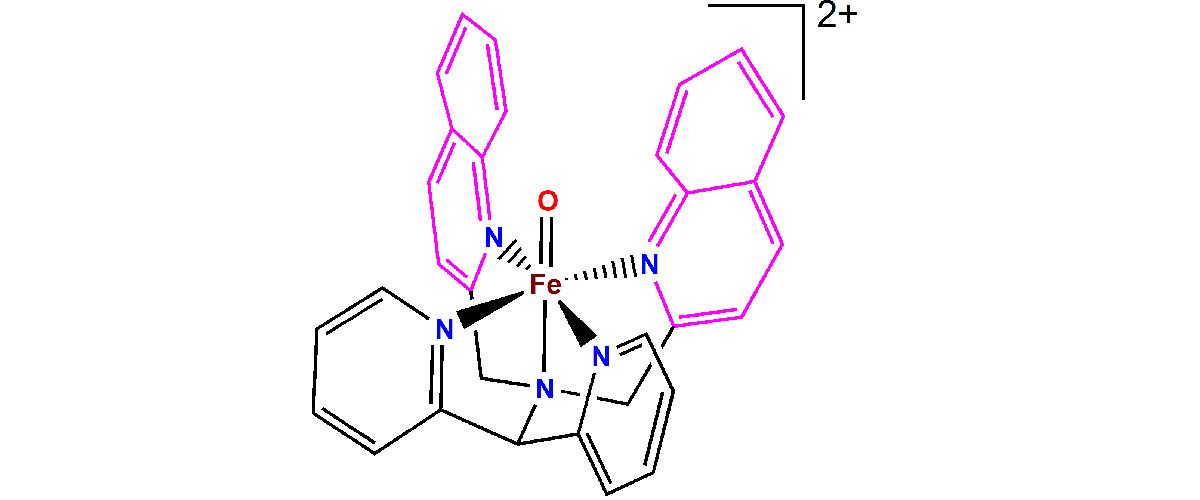
Chemists are actively working on synthesizing small molecules that replicate the reactions achieved in living systems by non-heme enzymes. An example of such a molecule is shown in Figure 1, for which the chemical reactivity was found to be tunable by modifying the ligands attached to the ferryl unit, the [Fe=O]2+ core of the complex.
Why is this important?
Iron (Fe) is the most important metal in biology and takes many forms in living systems. Relatively well known are the heme proteins and enzymes, such as the oxygen transport protein hemoglobin and the liver enzyme cytochrome P540, which detoxifies chemicals that enter the bloodstream. Less well known are the non-heme enzymes that also perform important biological functions.
Improving our understanding of non-heme enzymes will enable two advances: (1) the synthesizing of chemicals using “greener” technology (low-temperature and water-based, as in nature); and (2) the developing of antibiotics that specifically target these enzymes in pathogenic organisms.
Who did the research?
W. Rasheed1, A. Draksharapu1, S. Banerjee1, V.G. Young, Jr. 1, R. Fan2, Y. Guo2, M. Ozerov3, J. Nehrkorn3, J. Krzystek3, J. Telser4, L. Que, Jr.1
1University of Minnesota; 2Carnegie Mellon University; 3National MagLab; 4Roosevelt University
Why did they need the MagLab?
The ability to probe directly the [Fe=O]2+ active core of these complexes required the unique combination of photon energies and magnetic fields available at the MagLab. Data from these unique instruments enable comparisons among similar molecules to show subtle, but crucial, structural differences.
Details for scientists
- View or download the expert-level Science Highlight, Manipulating the ferryl tilt in a non-heme oxoiron(IV) complex that makes the complex a better oxidant
- Read the full-length publication, Crystallographic evidence for a sterically induced ferryl tilt in a non-heme oxoiron(IV) complex that makes it a better oxidant, in Angew. Chem. Int. Ed.
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1157490, NSF DMR-1644779); L. Que, Jr. (NSF CHE-1665391); Y. Guo (NSF CHE-1654060)
For more information, contact Jurek Krzystek.