What did scientists discover?
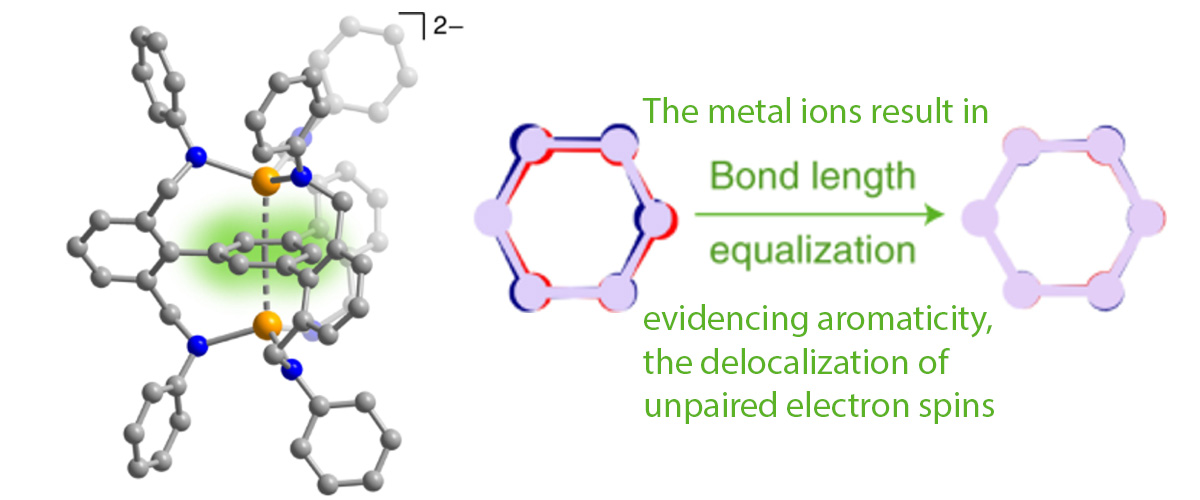
This study reports stabilization of the elusive benzene radical dianion with a magnetic ground state, that is, a doubly reduced form of the benzene molecule in which the two unpaired electrons align their magnetic moments, giving rise to a total spin S = ½ + ½ = 1 (triplet) ground state. High-field electron paramagnetic resonance (EPR) studies were employed in order to confirm the magnetic state of this unusual molecule.
Why is this important?
This study demonstrates how coordination chemistry can be leveraged to stabilize a desired electronic/ magnetic state in an organic molecule. Specifically, this approach enables isolation of a negatively charged (2-) benzene dianion in which the magnetic S = 1 (triplet) state—typically a high-energy excited state in such ring-like organic molecules—instead exists as the well-isolated molecular ground state. In turn, this enabled verification of a decades-old theoretical model predicting a delocalized nature of the unpaired electrons on the benzene ring.
Who did the research?
C. A. Gould1, J. Marbey2,3, V. Vieru4, D. A. Marchiori5, R. D. Britt5, L. F. Chibotaru4, S. Hill2,3 and J. R. Long1,6
1UC Berkeley; 2National MagLab; 3Florida State University; 4KU Leuven; 5UC Davis; 6LBNL
Why did they need the MagLab?
Measurements at multiple high magnetic fields and frequencies (a factor of four above commercial spectrometers) available at the MagLab were essential in order to deconvolute several contributions to the EPR spectra. In particular, separating the contributions from intra-molecular and inter-molecular interactions is impossible at low magnetic fields. In this way, analysis of spectra recorded at 13T and 371GHz provides information on the isolation of the S=1 state, without dependence on other unknown interactions.
Details for scientists
- View or download the expert-level Science Highlight, Isolation of a Triplet Benzene Dianion
- Read the full-length publication, Isolation of a Triplet Benzene Dianion, in Nature Chemistry
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1644779); J. R. Long (NSF CHE-1610226); S. Hill (NSF DMR-1610226); R. D. Britt (NIH 1R35GM126961); V. Vieru (Flemish Science Foundation)
For more information, contact Stephen Hill.