What did scientists discover?
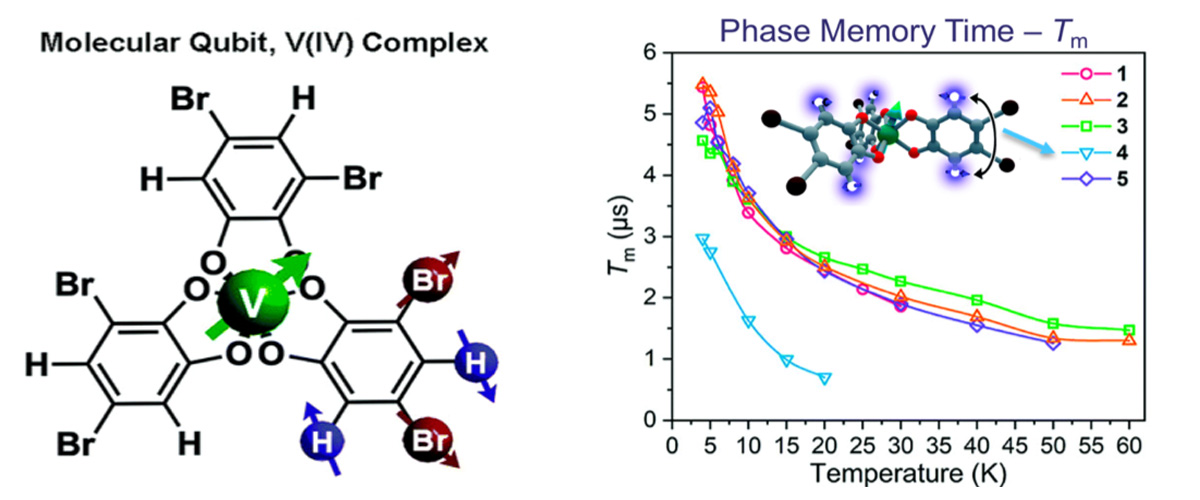
This study focuses on molecules containing transition metals that possess a single unpaired electron spin, for example, vanadium(IV). Such systems are of potential interest for next-generation quantum technologies. Electron spin resonance studies demonstrate that patterning of the organic groups on the periphery of the molecule with different atomic nuclei, e.g., protons (H) and bromine (Br) provides a handle on the quantum memory time, Tm, of the vanadium spin. Five different patterns of the peripheral organic groups are shown in the figures.
Why is this important?
Achieving chemical control of the quantum coherence of electron spins in transition metal containing molecules is an important goal in the development of future quantum technologies. Atomic nuclei represent a stubborn source of magnetic noise that can dramatically shorten quantum memory times, hence the desire to be able to control such interactions chemically.
Who did the research?
Cassidy Jackson1, Chun-Yi Lin1, Spencer Johnson1, Johan van Tol2 and Joseph Zadrozny1
1Colorado State University, Fort Collins; 2National MagLab, Tallahassee
Why did they need the MagLab?
Many factors influence the quantum memory times (Tm) of electron spins in molecules. This study sought to isolate the influence of the atomic nuclei on the electron spin coherence, which necessitates measurements at high magnetic fields where the nuclear contribution dominates.
Details for scientists
- View or download the expert-level Science Highlight, Nuclear Spin Patterning Controls Electron Spin Coherence
- Read the full-length publication, Nuclear-spin-pattern control of electron-spin dynamics in a series of V(IV) complexes, in Chem. Sci.
Funding
This research was funded by the following grants: G.S. Boebinger (NSF DMR-1157490, NSF DMR-1644779); J. M. Zadrozny (CHE-1836537, ACS PRF-60033-DNI3)
For more information, contact Stephen Hill.