In 1959, the famous physicist Richard Feynman gave a talk titled "There's Plenty of Room at the Bottom." Feynman's message to his peers was that the nano-world — the creation and control of matter at the scale of billionths of a meter — had barely been explored.
More than 50 years later, nanoscience has exploded. Universities around the world have launched nanoscience centers and initiatives; the United States funds a National Nanotechnology Initiative to the tune of more than $1 billion a year. More than 1,600 nanotechnology-based consumer products have hit the market, from high-tech coatings and sunscreens to catalysts and artificial tissue components.
And yet, a talk with Feynman's title would still be apt. In fields from electronics to data storage to medicine, many scientists feel that nanotechnology's potential is only starting to be realized.
"It's a vast field, and there's still new basic research coming out," said Vivien Zapf, a physicist at the National High Magnetic Field Laboratory. "Especially the ability to sense and manipulate individual atoms is a whole other field."
Scientists haven't always had the tools to study small-scale matter with the required precision. Ironically, studying the world's smallest constituents often requires some of the largest and most sophisticated hardware. (Just think of the 27-kilometer, $10 -billion Large Hadron Collider needed to discover the subatomic Higgs boson.)
The same is true for nanoscience. Some of the field's most cutting-edge work is now being done in high magnetic field labs around the world, where clever uses of the world's strongest magnetic fields are teasing out microscopic matter's secrets.
Magnetism for miniaturization
In physics, researchers are probing different kinds of molecules for properties that will lead to the next generation of electronics, even quantum computers.
In the hot field of spintronics, for example, scientists are looking for ways to use the "spin state" of electrons (or whether an electron is oriented up or down) as the ones and zeros of binary code.
Top: Zapf’s research was conducted in this world-record 100-tesla pulsed field magnet. (Credit: Dave Barfield); Bottom: Vivien Zapf. (Credit: Richard Sandberg)
At the National MagLab's Pulsed Field Facility, physicist Vivien Zapf is leading a team down a different path to nano-level electronics: multiferroics.
In multiferroic materials, one behavior can be used to control another, akin to the way electricity can generate magnetism and vice versa. But in multiferroics, that coupling is between magnetism and something called ferroelectricity. And just as magnetism and electricity help make the modern world go round, so too, some physicists believe, will the pairing of magnetism and ferroelectricity help the world of the future go round.
In ferroelectric molecules, the distribution of electric charge is uneven — one side is more positive, the other more negative. If you apply an electric field to it, you can get that polarity to flip. So ferroelectricity is a way of moving electric charges around, and in that sense is like electricity. However, ferroelectricity is a lot greener.
"Unlike spintronics, you're using voltages instead of electric current, so you reduce the power consumption," Zapf said.
That means ferroelectricity, when paired with magnetism in multiferroics, could pave the way to smaller, more powerful electronic devices that are far more energy-efficient than could be achieved with spintronics.
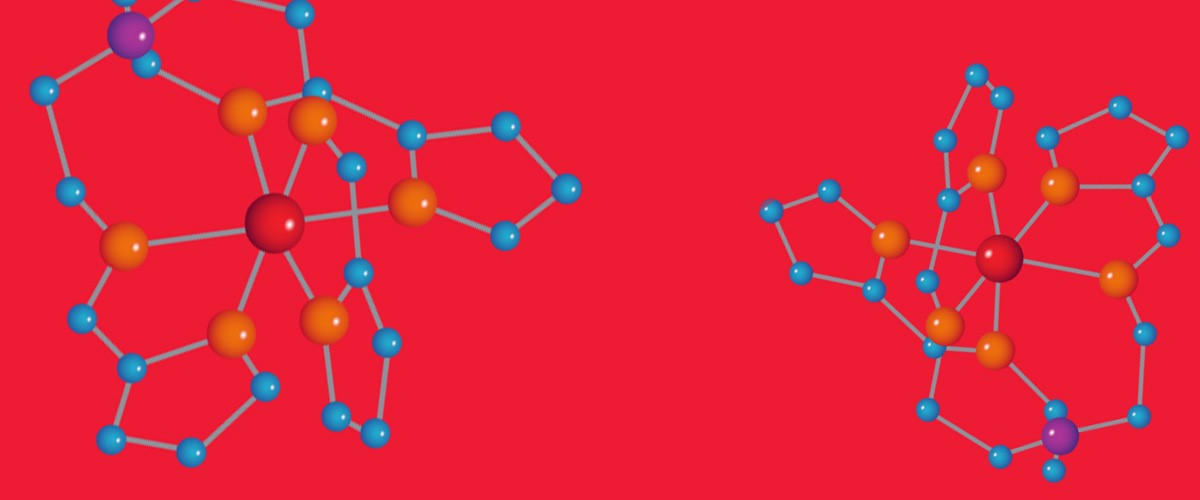
To that end, Zapf and her collaborators, including National MagLab physicist Shalinee Chikara, try to create multiferroic molecules. They believe they have found a promising candidate: a molecular magnet featuring a manganese atom surrounded by rings containing carbon and nitrogen — or MnIII(pyrol)3(tren), if you want to get technical.
By putting the molecule in very high magnetic fields up to 65 teslas, the scientists cause the electron spins to transition to a different state.
It's a new way to pursue multiferroics.
"Instead of thinking about which way the spins are pointing, you actually change their size," Zapf said. "Which means you're changing the configuration of electrons within one atom so that, overall, the atom ends up with a different degree of magnetism than it had before." That change in magnetism triggers the ferroelectricity, making the material multiferroic.
There's something else special about Zapf's molecule: While the majority of work in multiferroics has focused on inorganic materials (they don't contain carbon), Zapf's group is one of a few that studies materials with organic molecules, which do contain carbon. Their success opens up hundreds of thousands of similar organic materials for previously unexplored multiferroic effects, Zapf said.
"That has us pretty excited," Zapf said. "These hybrid inorganic-organic materials are a new route to designing magnetism."
Zapf may never have pursued this promising line of research if she hadn't decided to venture outside her discipline and attend a chemistry conference a few years ago. There, she learned about spin state transitions — an area most physicists don't know much about.
"By letting physics and chemistry intersect, we get access to this new way of approaching multiferroics," Zapf said.
With her chemist colleagues from around the world, Zapf is cracking the door on a host of possible applications. Multiferroics could lead to new, highly sensitive magnetic sensors and new designs for small-scale, high-frequency devices such as antennas, power transformers or MRI magnets.
But figuring that out is someone else’s job, she said.
"There are people who specialize in applications. I'm more into, 'Let's design crazy new ways to make ferroelectrics talk to ferromagnets,'" she said. "I'm one of the people who feeds them the new ideas and new approaches."
Zapf's colleagues on a recent paper include National MagLab physicists Shalinee Chikara and John Singleton, chemists Nathan Smythe and Brian Scott of Los Alamos National Laboratory, theorists Shizeng Lin and Cristian Batista (University of Tennessee), and a student project at Harvey Mudd College with Jim Eckert and Elizabeth Krenkel.
By Gabriel Popkin
This story was originally part of a series of stories called Going Nano about how good science can come in tiny packages — all with the aim of solving really big problems. To read the other stories in the series, see Nanocages May Hold Key to Advances in Health Energy and Nano Drug Delivery is Rocket Science.