Who is the scientist?
Lissa Anderson, a chemist in the MagLab's Ion Cyclotron Resonance (ICR) Facility, recently conducted experiments in collaboration with researchers from other institutions. External scientists frequently enlist the help of our on-site magnet gurus when they can't make it to the lab to conduct the experiment in person. Anderson specializes in biological applications of ICR.
What is going in the magnet?
Short Answer: Hemoglobin proteins purified from patient blood.
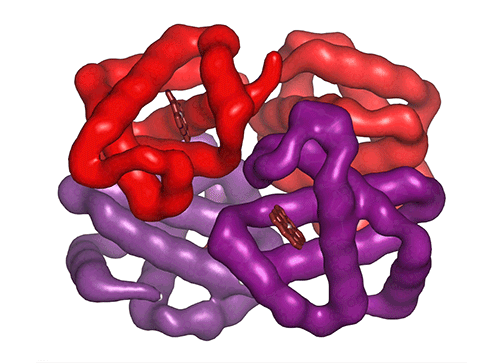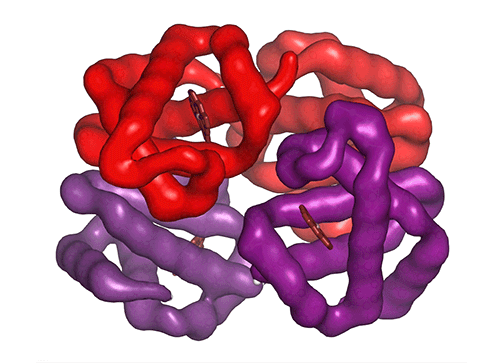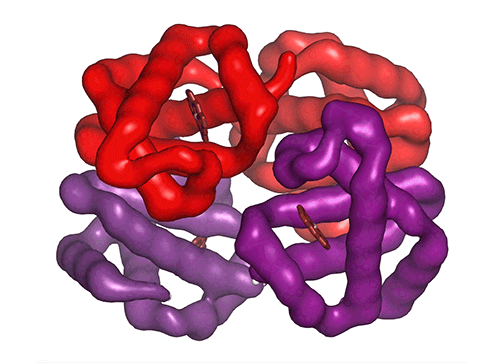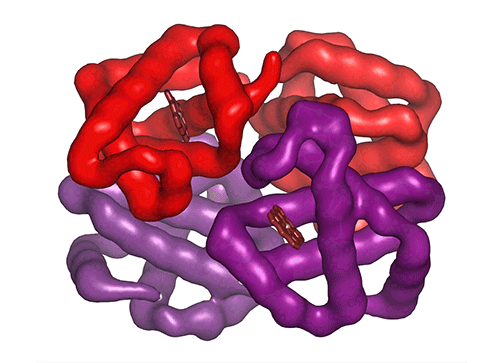
Each of the four hemoglobin subunits (α and β chains) can bind one oxygen molecule. Red blood cells transport this oxygen from the lungs to tissues around the body. Some hemoglobin variants can mutilate the protein structure, influencing oxygen binding and transport efficiency.
(Animation credit: Abigail Engleman)
Longer Answer: Researchers were trying to identify new kinds of hemoglobin (Hb) variants, which can alter the protein's structure and/or function.
Hemoglobin are proteins in blood whose job it is to carry oxygen from your lungs to tissues throughout your body. Like all other proteins, it begins life as a recipe known as DNA. A mutation in the DNA can result in variation in the protein. In fact, an estimated 7 percent of people around the world are affected by naturally occurring Hb variants. Although some of these variants are insignificant, others interfere with the shape of the Hb protein and/or its ability to carry oxygen, which can result in diseases such as sickle cell anemia. This is bad news for us oxygen breathers.
Scientists have already found over 1,000 variants using traditional methods such as gene sequencing. But at the MagLab, a new, powerful tool is being used to identify more. The world-record 21-tesla ICR mass spectrometer offers researchers a new, highly efficient analysis method for studying the proteins that make up hemoglobin. This provides detailed results and higher accuracy, increasing scientists’ ability to detect variants.
Hemoglobin proteins comprise four subunits, each made up of one of two kinds of amino acid chains. Scientists know exactly what these subunits look like. And because each constituent amino acid has a unique molecular mass, they know precisely what a normal subunit weighs. The 21 T instrument is so sensitive that it can weigh these subunits and can determine when the weight is a little higher or lower than it should be. When that happens it means that, due to some change in the genetic code, at least one amino acid that was in the original recipe has been substituted for another.
Not only can they identify an Hb variation this way, but they can also fragment the abnormal subunit, analyze it in the instrument, and tell exactly which in a long chain of nearly 150 amino acids was substituted, what was put in its place, and exactly where in the chain that substitution occurred.
Why do I care?
ICR technology improves researchers’ ability to detect hemoglobin variants, a critical first step in understanding the physiological effects associated with each one. This research could help identify as yet undiscovered variants and shed light on symptoms that patients with Hb mutations may experience.
Story by Abigail Engleman


