Chromatography, literally "color writing," was first used by Russian botanist Mikhail Tswett, who used columns of calcium carbonate to separate plant pigments such as cholorphyll and carotenoids in 1901.
Later, a Nobel Prize was awarded to Archer John Porter Martinand Richard Laurence Millington Synge for "invention of partition chromatography" in 1952. Efforts during the past several decades have focused on the chromatography instrumentation, such as pumps, autosamplers, and detectors, as well as on the development of columns for faster separation and better resolution. Liquid chromatography is one of the most important techniques for the separation of complex mixtures.
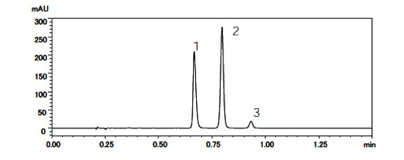
Liquid chromatography separates a sample into its individual component in the following steps. A small volume of liquid sample is injected and loaded onto a tube packed with porous particles (stationary phase). The components of the sample are transported along the packed tube (column) with a liquid (mobile phase) moved by gravity or high pressure. The sample constituents are separated based on their different affinity for the mobile or stationary phase. On eluting from the column, the detected component signal vs. time constitutes a "liquid chromatogram" (See Figure 1).
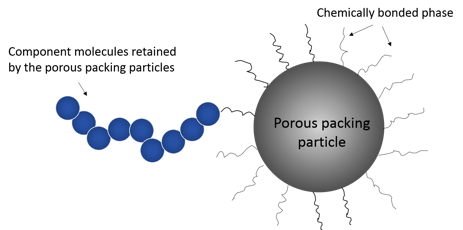
Liquid chromatography separation is based on affinity of the analyte for both the stationary phase and mobile phase. Silica is one of the most popular adsorbent materials for liquid chromatography. Silica particle sizes generally range between 3 and 50 microns, and the particle pore size ranges between 100-1000 Angstrom. The porous silica particles in the column usually have a chemically bonded phase (hydrophobic alkyl chains) on their surface that interacts with the components through chemical bonding (Figure 2). The three common chain lengths are C4, C8 and C18. C4 is mostly used for proteins whereas C8 and C18 are used for peptides or small molecules. Proper selection of mobile phase is also crucial for a successful separation. The main requirement for the mobile phase is that it dissolves the components up to the concentration suitable for the detection. The type of mobile phase may have a great effect on the retention time in separation as well as the ionization of the components in downstream detection. The pH of the mobile phase can have an important role on the retention of a component and can affect the selectivity.
Depending on the choice of stationary and mobile phase, four major separation modes are used to separate most compounds.
Reversed-phase chromatography employs a non-polar stationary phase and a polar mobile phase. Therefore, hydrophobic molecules in the polar mobile phase tend to adsorb to the stationary phase and the hydrophilic molecules in the polar mobile phase will be transported along the column and elute first. Mixtures of water or aqueous buffers and organic solvents are used to elute components from a reversed-phase column. Gradient elution, in which the water-solvent composition changes as a function of time, is often used to separate a sample containing a wide range of components. Today, reversed-phase chromatography is the most widely used type of liquid chromatography.
In normal phase chromatography, the stationary phase is polar and the mobile phase is non-polar. The stationary phase is generally silica or organic moieties with cyano and amino functional groups and the mobile is hexane or heptane mixed with a slightly more polar solvent such as isopropanol, ethyl acetate, or chloroform. In normal phase chromatography, the least polar compounds elute first and the most polar compounds elute last. Normal phase chromatography is very useful to separate water-sensitive compounds, geometric isomer, cis-trans isomers, and chiral compounds.
In ion exchange chromatography, the stationary phase contains ionic groups, for example, sulfonic or tetraalkylammonium, and the mobile phase is an aqueous buffer. The component molecule is retained by the stationary phase by coulombic attraction. This type of chromatography can be further divided to two categories: cation exchange chromatography retains positively charged cations and the anion exchange chromatography retains negatively charged anions. Ion exchange chromatography is most commonly used for to separate inorganic and organic anions and cations in aqueous solution.
In size exclusion chromatography, there is no chemical interaction between the sample molecules and the stationary phase. Instead, molecules are separated depending on their size relative to the pore size of the stationary phase. Largest molecules elute first and smallest molecules (which can permate into the pores) elute last. Size exclusion chromatography is also known as gel-filtration chromatography and is widely used to separate polymer and proteins.
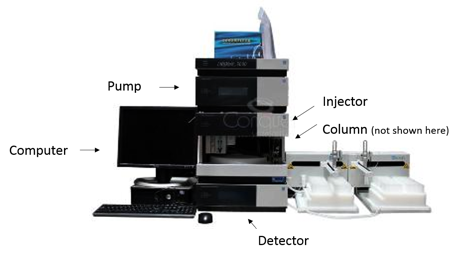
Liquid chromatography that utilizes small packing particles and a relatively high pressure is referred to as high performance liquid chromatography (HPLC). A basic HPLC system consists of a pump, injector, column, detector, and a computer. Figure 3 shows the Dionex Ultimate 3000 HPLC system used at NHMFL.
The most common HPLC detection is based on ultraviolet absorption or mass spectrometry. In ultraviolet absorption, an ultraviolet light beam is directed through a flow cell, and a sensor measures the intensity of light passing through the cell. In mass spectrometry, detection is based either on mass of the intact analyte (MS1) or on its fragments (Tandem MS) produced by electron transfer dissociation, electron capture dissociation, or collision-induced dissociation. Liquid chromatography - mass spectrometry is a powerful technique for characterization of biopolymers.
This technique can be used with the following instruments:
Explore our magnet schedule to see what exciting research is happening on our stellar fleet of instruments right now.
T. T. Lam, et al, Mapping of protein:protein contact surfaces by hydrogen/deuterium exchange, followed by on-line high-performance liquid chromatography-electrospray ionization Fourier-transform ion-cyclotron-resonance mass analysis, Journal of Chromatography A 982(1), 85-95 (2002) Read online.
For more information please contact Lissa Anderson.
Last modified on 08 August 2023